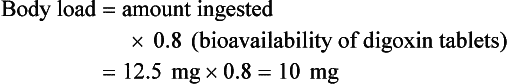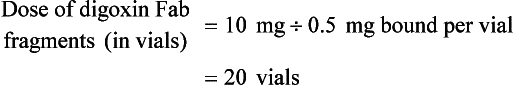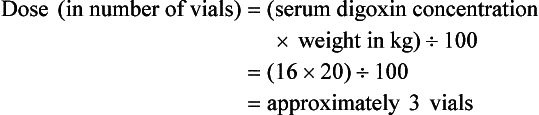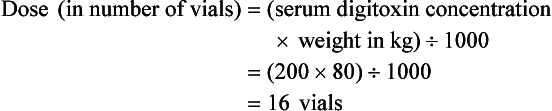Chapter 152 Cardiovascular drugs are a common cause of poisoning in the United States; in 2010, 97,336 exposures to cardiovascular drugs were reported to U.S. Poison Control Centers.1 Cardiovascular drugs are the second most common cause, accounting for more than 10%, of all poisoning deaths in the United States. Of the scores of cardiovascular drugs, three classes—cardioactive steroids (primarily digoxin), beta-adrenergic blockers, and calcium channel blockers—account for the majority of fatalities. Digoxin is derived from the Balkan foxglove plant, Digitalis lanata (Fig. 152-1), and the trade name for digoxin (Lanoxin) is derived from the Latin name of this plant.2 Digitoxin, which is no longer in clinical use, comes from Digitalis purpurea.2 Although William Withering was the first to describe the medicinal use of the foxglove plant,3 the ancient Egyptians reference medicinal use of foxglove. Despite centuries of experience with digitalis, chronic and acute poisonings still occur. Controversy about the therapeutic benefit of cardioactive steroids is not new. Benjamin Rush wrote in 1797, “I suspect the cases in which [digitalis preparations] were useful to have been either so few or doubtful and that the cases they had done harm were so much more numerous and unequivocal as justly to banish them from the Materia Medica.”4 Medication errors and toxic effects account for the most common causes (44%) of preventable iatrogenic cardiac arrests. Digoxin is used therapeutically (1) to increase the force of myocardial contraction to increase cardiac output in patients with heart failure and (2) to decrease atrioventricular (AV) conduction to slow the ventricular rate in atrial fibrillation. The basis for its first effect is inhibition of membrane sodium-potassium–adenosine triphosphatase (Na+,K+-ATPase), which increases intracellular sodium and extracellular potassium concentrations. This increase in intracellular sodium concentration results in dysfunction of the sodium-calcium ion exchanger, which normally extrudes intracellular calcium after systole. This subsequent increase in intracellular calcium concentration results in a larger amount of calcium pumped into the sarcoplasmic reticulum, so that on calcium-induced calcium release during subsequent action potentials, a larger amount of calcium is released into the cell, causing a more powerful contraction and thus increased cardiac output. Any molecule with this effect is classified as a cardioactive steroid. Cardiac glycosides (such as digoxin) are merely cardioactive steroids with additional sugar moieties attached to their steroid nucleus. At therapeutic doses, the effects of digoxin on serum electrolyte levels are minimal. With toxic levels, digoxin paralyzes the Na+,K+-ATPase pump, potassium cannot be transported into cells, and serum potassium concentration can rise as high as 13.5 mmol/L.5 Digoxin also exerts three primary effects on Purkinje fibers: (1) decreased resting potential, resulting in slowed phase 0 depolarization and conduction velocity; (2) decreased action potential duration, which increases sensitivity of muscle fibers to electrical stimuli; and (3) enhanced automaticity resulting from increased rate of phase 4 repolarization and delayed afterdepolarizations. These mechanisms account for an increase in premature ventricular contractions, the most common manifestation of digoxin toxicity. At toxic extremes, these effects result in a dangerous sensitivity to mechanical and electrical stimulation. Interventions with pacemaker wires, catheters, and cardioversion can result in asystole, ventricular tachycardia, and ventricular fibrillation.6 Unlike most cardiovascular drugs, digoxin can produce virtually any dysrhythmia or conduction block, and bradycardias are as common as tachycardias (Box 152-1). Unfortunately, none is unique to digoxin, and because they can all occur in the setting of ischemic and other heart disease, digoxin toxicity remains a clinical rather than an electrocardiographic diagnosis. The volume of distribution (Vd) of digoxin is 5 L/kg for adults but varies from 3.5 L/kg in premature infants to 16.3 L/kg in older infants.7 This indicates that only a small fraction of digoxin remains in the intravascular space, and the drug is highly concentrated in cardiac tissue. The myocardial-to-serum ratio at equilibrium ranges from 15 : 1 to 30 : 1. The Vd for digitoxin is only 0.5 L/kg, giving it a different pharmacokinetic profile. Multiple drugs and disease states can negatively alter absorption, Vd, protein binding, and elimination and render the heart more susceptible to digoxin toxicity. The factors listed in Box 152-2 are especially important risk factors in chronic intoxication. The symptoms and signs of chronic digoxin toxicity are nonspecific. The most common symptoms, in more than 80% of cases, are nausea, anorexia, fatigue, and visual disturbance, but a variety of gastrointestinal, neurologic, and ophthalmic disturbances also occur (Box 152-3). Digoxin intoxication should be considered in any patient receiving maintenance therapy who has consistent symptoms, especially with new conduction disturbances or dysrhythmias. There are significant differences between acute and chronic toxicity (Table 152-1). Chronic poisoning has an insidious onset and is accompanied by a higher mortality rate that is probably due in part to underlying heart disease and chronic accumulation of the toxin. In cases of chronic intoxication, the LL50 (the level with a 50% mortality) is only 6 ng/mL.8 The LL50 for acute intoxication is not known, but it is certainly much higher, especially in children. The association of hyperkalemia with acute toxicity is obvious given the mechanism of digoxin; either hypokalemia or hyperkalemia may occur with chronic toxicity. Table 152-1 Chronic versus Acute Digitalis Intoxication Diagnosis and management rely heavily on serum digoxin levels, but it is the steady state, rather than peak concentration, that correlates with tissue toxicity and is used to calculate antidote dosages. Peak concentrations after an oral dose of digoxin occur in 1.5 to 2 hours, with a range of 0.5 to 6 hours.9 Steady-state serum concentrations are not achieved until after distribution, or 6 to 8 hours after a dose or overdose, and may be only one fourth to one fifth of the peak concentration. The ideal serum digoxin concentration for patients with heart failure is considered to be 0.7 to 1.1 ng/mL,10 although laboratory “normals” are often reported up to 2.0 ng/mL. Serum steady-state digoxin levels of 1.1 to 3.0 ng/mL are equivocal; that is, levels as low as 1.1 ng/mL have been associated with increased mortality,11 and patients with levels up to 3.0 ng/mL can be asymptomatic.12 The incidence of digoxin-incited dysrhythmia reaches 10% at a level of 1.7 ng/mL and rises to 50% at a level of 2.5 ng/mL. Determination of a level too soon after the last maintenance dose falsely suggests toxicity, especially in cases of chronic intoxication, in which significant morbidity and mortality can occur at levels of 2 to 6 ng/mL.10 After an acute massive overdose in a patient who is rapidly becoming symptomatic, however, it may be impractical to wait 6 to 8 hours for the first reading.13 It is unlikely that early levels exceeding 10 to 20 ng/mL will fade to clinical insignificance at 6 to 8 hours after ingestion. The mortality rate before Fab fragment therapy was 23% despite all of the interventions described.14 Fab fragment treatment is well established in both chronic and acute poisonings, with a 90% response rate.15 Nonresponders usually receive too little antibody or receive it too late. Other nonresponders are compromised by underlying heart or multisystem disease. Digitalis antibodies are derived from sheep, but allergic reactions occur in less than 1% of cases.15 Reactions have included erythema, urticaria, and facial edema, all of which are responsive to the usual treatment. Other expected reactions when Fab fragments neutralize digitalis include hypokalemia, exacerbation of congestive heart failure, and increase in ventricular rate with atrial fibrillation. Two Fab fragment preparations were previously available; however, Digibind has been discontinued, leaving DigiFab as the only available product in the United States. If vials of Digibind are still available at a given institution, they require a 0.22-µm membrane filter for proper use; such a filter is not required for DigiFab. Fab fragment treatment is best reserved for cases of serious cardiovascular toxicity rather than for routine or prophylactic administration with higher than expected serum levels. In acute poisoning, antibody treatment should be used for a serum potassium level above 5.0 mEq/L or unstable dysrhythmias, such as symptomatic sinus bradycardia, ventricular dysrhythmias, or second- or third-degree heart block unresponsive to atropine. Although toxicity increases with greater body load, there is no clear correlation with amount ingested, especially in children, and many patients with large ingestions or high serum levels become only mildly symptomatic.8 Fab fragment therapy should be used before transvenous pacing, which carries significant risk. The median time to initial response is 19 minutes after completion of the Fab infusion, but complete resolution of digitalis toxic rhythms may require hours.16,17 Late administration of Fab fragments has resuscitated 54% of patients who have suffered cardiac arrest.18 This antidote should be considered whenever hemodynamic compromise attends a digitalis toxic dysrhythmia or heart block (Box 152-4).19 Current formulas for calculation of DigiFab doses are found in the package insert. There are at least three approaches. The first is empirical. A patient has a history of digitalis ingestion, consistent symptoms, and life-threatening dysrhythmias. There is no time to assess serum digoxin levels, and 10 vials should be administered over 30 minutes for the average acute ingestion, 4 to 6 vials for the average chronic ingestion. In cardiac arrest, 20 vials can be administered undiluted by intravenous bolus. The second approach uses a simple calculation when the ingested dose is known with reasonable certainty. One vial of Digibind or DigiFab contains 38 mg or 40 mg, respectively, of Fab fragments, which bind 0.5 mg of digoxin or digitoxin (Box 152-5). A third approach is to base the dosage on the steady-state serum digoxin or digitoxin level after 6 to 8 hours (Boxes 152-6 and 152-7). Because most assays measure both bound and unbound drug, digoxin levels will be elevated for up to 1 week, with values often greater than 100 ng/mL once Fab fragments have been administered. Newer methods can measure free digoxin, but it is more meaningful to follow the patient clinically. In acute poisoning, serum potassium concentration may begin to rise rapidly within 1 to 2 hours of ingestion. Potassium should be withheld, even if mild hypokalemia is measured initially. The initial serum potassium concentration may in fact be a better predictor of mortality than the initial digoxin concentration. In a study of 91 patients with acute digoxin poisoning, nearly 50% of the patients with serum potassium concentrations between 5.0 and 5.5 mmol/L died. No patients with a potassium level of less than 5.0 mEq/L died, and all 10 patients with serum potassium concentrations exceeding 5.5 mmol/L perished.20 Because of these findings, a serum potassium concentration greater than 5 mmol/L alone warrants treatment with Fab fragments. Even with Fab fragments, severe hyperkalemia should be treated with intravenous administration of glucose, insulin, and sodium bicarbonate as needed. The decision to administer calcium to patients with hyperkalemia and digoxin poisoning represents a clinical dilemma. Classic teaching is that in the setting of the increased intracellular calcium concentration from digoxin poisoning, administration of exogenous calcium will result in “stone heart” from excessive intracellular calcium.21 This concept has been in the literature since 1927,22 based on animal studies.23 Documented cases of cardiac arrest after calcium administration are exceedingly rare in the literature, and the temporal relationship is dubious.24 More recent data indicate that the intravenous administration of calcium can be safe for hyperkalemia in the setting of digoxin toxicity.25 Unequivocally, however, the best treatment of hyperkalemia due to acute digoxin toxicity is Fab fragments. The treatment of hyperkalemia in the setting of chronic digoxin toxicity and renal failure is less clear; however, the evidence that calcium salts will be harmful is dubious at best. Calcium salts should be administered during several minutes through a secure peripheral intravenous site or through a central venous catheter. Indications are identical to those for hyperkalemia, including but not exclusive to a widened QRS duration on the electrocardiogram. A reasonable starting dose would be 3 g of calcium gluconate or 1 g of calcium chloride. Calcium salts should be followed immediately with other pharmacologic measures to lower serum potassium concentration. Many patients receiving diuretic therapy are also magnesium depleted, even when the measured serum magnesium level is normal. If significant magnesium depletion is suspected (e.g., if electrocardiographic changes such as QTc prolongation are present), 1 to 2 g of magnesium sulfate can be given during 10 to 20 minutes (child: 25 mg/kg), followed by a constant infusion of 1 to 2 g/hr. Patients should be closely monitored for respiratory depression, which is usually preceded by progressive loss of deep tendon reflexes. Hypermagnesemia can exacerbate digitalis toxicity, but magnesium has been reported to reverse digoxin-induced tachydysrhythmias.26 It is prudent to infuse magnesium slowly and to stop the infusion if heart block or bradycardia develops. Avoid magnesium in patients with renal failure. The role of magnesium in bradydysrhythmias and conduction blocks is less clear but probably dangerous because hypermagnesemia can impair impulse formation and AV conduction. Transvenous pacing has been a mainstay of treatment for several decades, but the catheter may induce ventricular tachydysrhythmias in a myocardium made irritable by digoxin. Iatrogenic accidents of cardiac pacing are frequent (14 of 39, 36%) and often fatal (5 of 39, 13%).27 We recommend avoidance of transvenous pacing unless external pacing fails. Pacing usually is required only temporarily while waiting for Fab fragments to take effect. Cardioversion for tachydysrhythmia also may be hazardous in the setting of digoxin intoxication. If it is deemed necessary, such as when the tachydysrhythmia is thought to be causing severe hypotension, we recommend use of lower than usual energy settings, such as 25 to 50 J, although there is no proof that this is less hazardous. Phenytoin and lidocaine are believed to be the safest of the antidysrhythmic drugs for control of tachydysrhythmias in the setting of digoxin toxicity. Indications include unstable tachydysrhythmias when Fab fragments are unavailable and unstable tachydysrhythmias that occur while waiting for Fab fragments to take effect. Phenytoin may enhance AV conduction. Phenytoin has been infused at 25 to 50 mg/min to a loading dose of 15 to 20 mg/kg; it may also be administered in 100-mg boluses every 5 minutes until arrhythmias improve or 1000 mg is administered. Lidocaine can be given initially at a dosage of 1 to 1.5 mg/kg during several minutes, followed by an infusion of 1 to 4 mg/min (30-50 µg/kg/min).28 Most other cardiac drugs (isoproterenol, procainamide, amiodarone, beta-blockers, calcium antagonists) may worsen dysrhythmias or depress AV conduction. Digoxin immune Fab fragments are the preferred therapy for dysrhythmias.
Cardiovascular Drugs
Cardioactive Steroids (Digoxin)
Principles of Disease
Clinical Features
CHRONIC
ACUTE
Higher mortality (LL50 6 ng/mL)
Lower mortality
Ventricular dysrhythmias more common
Bradycardia and atrioventricular block more common
Usually elderly patients
Usually younger patients
Often need Fab fragment therapy
Often do well without Fab (Caution: many exceptions)
Underlying heart disease increases morbidity and mortality
Absence of heart disease decreases morbidity and mortality
Diagnostic Strategies
Management
Electrolyte Correction
Pacing
Phenytoin and Lidocaine

Full access? Get Clinical Tree


Cardiovascular Drugs
Only gold members can continue reading. Log In or Register to continue