I. INTRODUCTION
A. Scope of the Problem in the United States
1. Burns are a major source of morbidity: approximately two million burns occur per year. Burns result in more than 60,000 hospitalizations and nearly 6,000 deaths per year. Total health care expenditures approach 4 billion dollars per year. Recent reports demonstrate a 50% decline in burn-related deaths and hospital admissions over the last 20 years.
2. Large-burn patients should be cared for as other critical care patients—providers should make sure to assess all organ systems. Though formulas for fluid resuscitation are well known, it is important to assess the resuscitative progress similar to the resuscitative efforts of other critically ill patients. While, critical illness in burned patients is most often caused by sepsis, burn wound sepsis is no longer seen as frequently due to aggressive early debridement. In fact, pneumonia is now most common cause of sepsis in burn patients.
B. The first steps of the overall critical care management plan are the initial assessment and resuscitation, followed by early wound excision and biologic closure either with autograft (if available) or allograft within 96 hours. Subsequently definitive wound closure is achieved and rehabilitation can begin. Throughout the execution of this plan, the physiologic effects of burn injury must also be kept in mind. They are as follows:
1. Early hypodynamic “ebb” phase: Early hours after injury with hypodynamic state requiring aggressive critical support.
2. Hyperdynamic “flow” phase: After first few hours, patient will develop increased cardiac output, increased muscle catabolism, and decreased peripheral vascular resistance.
3. Massive capillary leak thought to result from burn wound inflammatory mediators.
4. Postresuscitative phase: Volume requirements often decline after the first 24 hours and capillary leak decreases. Patient then has a hyperdynamic phase with fever and increased protein catabolism. Inflammatory mediators such as TNF-α, IFN-γ, IL-6, and IL-10 are released.
II. COMPONENTS OF PRIMARY AND SECONDARY SURVEY IN BURN PATIENTS
During their initial evaluation, burn patients should have a primary and secondary survey according to advanced trauma life-support guidelines. The components of this are the following:
A. Airway. Objective measurements include respiratory rate, respiratory effort, breath sounds, skin assessment, and intraoral and intranasal examination for soot and mucosal inflammation. It is important to establish definitive airway before transport, especially if large fluid requirements are estimated (>30% TBSA). Even without airway injury, patients develop progressive mucosal edema from massive inflammation and fluid requirements. For airway burns, it is crucial to evaluate for burns of the face, eyes, neck, and nasopharynx, including soot in these regions. Get a detailed history of where the patient sustained their burn as inhalation injuries rarely occur in nonenclosed spaces. Assess if patient was exposed to chemicals or carbon monoxide. If carboxyhemoglobin levels are >20%, consider hyperbaric oxygen (only if patient is stable enough) or 100% FIO2. When intubating, make sure an airway assessment is performed initially. If patient has difficult anatomy, it is important to have fiberoptic setup, bougie, and surgical consultation in case a surgical airway is needed. The patient’s hemodynamics should be assessed prior to intubation. Typical induction medications can include propofol (make sure patient is not hypotensive) or etomidate (may get delayed adrenal insufficiency) and rocuronium as the paralytic (avoid succinylcholine). All of these induction agents cause hypotension, so providers should be ready with a vasopressor such as IV neosynephrine. Newer studies demonstrate ketamine might represent alternative with less hypotensive effects.
B. Breathing. Once patient is intubated, it is crucial to assess location of the tube through auscultation, visualization of moisture in the endotracheal tube, bilateral chest rise, and CO2 detection. Take care when securing the endotracheal tube around the head to prevent a potential tourniquet effect as the patient becomes edematous during resuscitation.
C. Circulation. It is important to have large-bore IV access with 16 or 18G peripheral IVs. It is better to place access in nonburned areas though this is not always possible. If the patient is going to require invasive monitoring, a central line should be placed under sterile conditions. If a PiCCO device (Pulsion Medical Systems AG, Munich, Germany) is used to monitor the patient, it is best to have central line in the internal jugular vein and the arterial line in the groin. This technology uses thermodilution to determine cardiac performance. Studies have demonstrated feasibility of this technology compared to pulmonary artery catheter. Blood pressure cuffs can be difficult to obtain an accurate read, making arterial lines useful.
D. Disability/Neurologic Assessment. Using the “AVPU” mnemonic, evaluate if patient is Alert, responsive to Verbal stimuli, responsive to Painful stimuli only, or Unresponsive, which might help assess if patient is hypoxic, hypercarbic, or altered. Obtain a baseline Glasgow Coma Score, and make sure there are no other injuries.
E. Exposure and Environmental Control. It is important to remove all clothing, especially if a chemical injury is sustained, and it is necessary to decontaminate (copious water irrigation until pH = 7) the patient prior to transport. Make sure to remove any jewelry that can create a tourniquet effect once swelling sets in.
III. BURN-SPECIFIC SECONDARY SURVEY
A. History: mechanism and timing of injury; was there chemical exposure and, if so, what are the side effects of this chemical; was the patient inside or outside; was there any risk of inhalation injury; neurologic status; extrication time; fluids and mediations given on scene and during transport; tetanus status; code status; and decision makers
B. Face: detailed orbital and periorbital exam. Electrical injury increases risk of glaucoma. Massive fluid resuscitation can cause intraocular compartment syndrome. Ophthalmology consult should be obtained if there is concern for corneal injury, and they should perform a fluorescein dye test. If conjunctival swelling, patient may not be able to close their eyelids. If this is the case, consider temporary tarsorrhaphy. Large-volume irrigation of globes with neutral pH solution is required, especially if chemical injury. Examine the nose for singed vibrissae. Perform an intraoral exam to look for swelling and carbonaceous sputum.
C. Neurologic: Abnormal neurological exam may indicate carbon monoxide exposure.
D. Chest: Assess chest compliance and if eschar is limiting chest excursion and decreasing compliance.
E. Genitourinary System: Early Foley catheter placement allows for close monitoring of urine output and therefore kidney perfusion. Deeply burned foreskin may affect penile perfusion.
F. Extremities: Important to examine all extremities (proximal and distal) for compartment syndrome. Unlike crush injury or electrical injury (caused by immobile fascia), flame and scald burns cause compartment syndrome by creating immobile skin layer. Most sensitive exam finding for compartment syndrome is pain with passive movement; however, this may not be possible in intubated patient. Important to assess distal two-point discrimination (normal <6 mm) and arterial pulses. By time there is a change in neurologic or vascular exam of the extremity, it is usually too late. If electrical injury, it is important to monitor serum creatine kinase (CK), and, in addition to escharotomy, patient will require fasciotomy to release deeper compartments.
G. GI: Patient may require abdominal escharotomy for large-volume resuscitation to avoid abdominal compartment syndrome. If patient develops abdominal compartment syndrome, it should be addressed by making sure adequate escharotomy is done, laying patient supine, making sure the patient has a nasogastric tube, treating pain, and starting a paralytic (not succinylcholine) if needed. If these measures fail, the patient will need a laparotomy, which has extremely high mortality in burn patients.
H. Initial Wound Evaluation: crucial to assess location and depth of all burns using a burn diagram. Rule of 9s estimate that surface area for each upper extremity is 9%, lower extremity 18%, anterior chest/abdomen 18%, back 18%, and head 9% (Fig. 34.1). Alternatively, you can use the patient’s palm, which represents 1% to estimate burn area. In children, the head is 18% and the lower extremities are each 14%. Burn wound often has three zones of injury (Fig. 34.2). Zone of coagulation has full-thickness burn through the epidermis and dermis. The zone of stasis can go on to heal or progress to become zone of coagulation. Zone of hyperemia is superficial and will go on to heal.
I. Burn Depth
1. Superficial partial thickness: Burn down to papillary dermis
2. Deep partial thickness: Burn down to reticular dermis
J. Laboratories and Radiographs
1. Carboxyhemoglobin if concern for inhalation injury (normal up to 3% in nonsmokers, up to 15% is normal in smokers)
2. Chest x-ray to assess placement of endotracheal tube
3. CBC, chemistries, LFTs, coags, T&S, ABG
IV. TOOLS TO HELP IN HEMODYNAMIC MONITORING
The provider should also consider the different tools to help in hemodynamic monitoring, including the following:
A. Central venous pressure trend can be used to assess progress of resuscitation, though the absolute numbers have limited value.
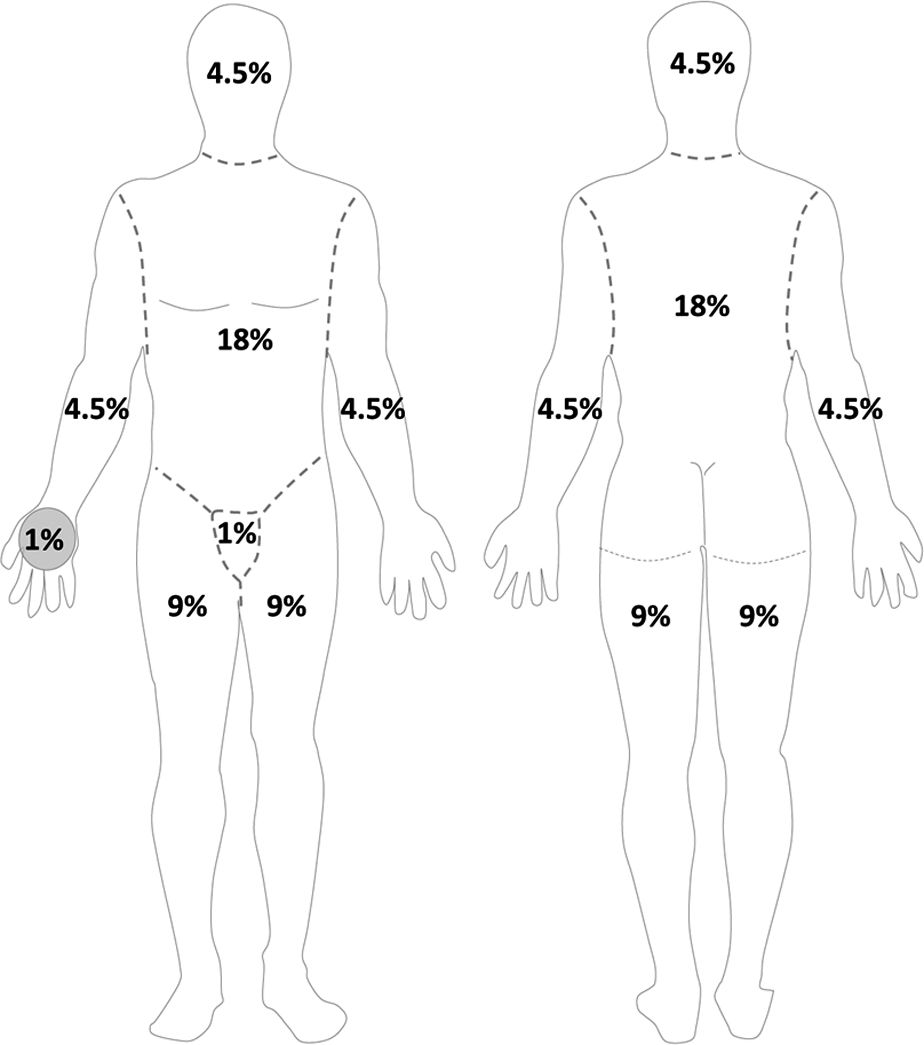
FIGURE 34.1 Schematic of “Rule of 9s” to estimate burn surface area in adults. Anterior (left panel) and posterior (right panel) views of body. Area of palm approximates 1% total body surface area.
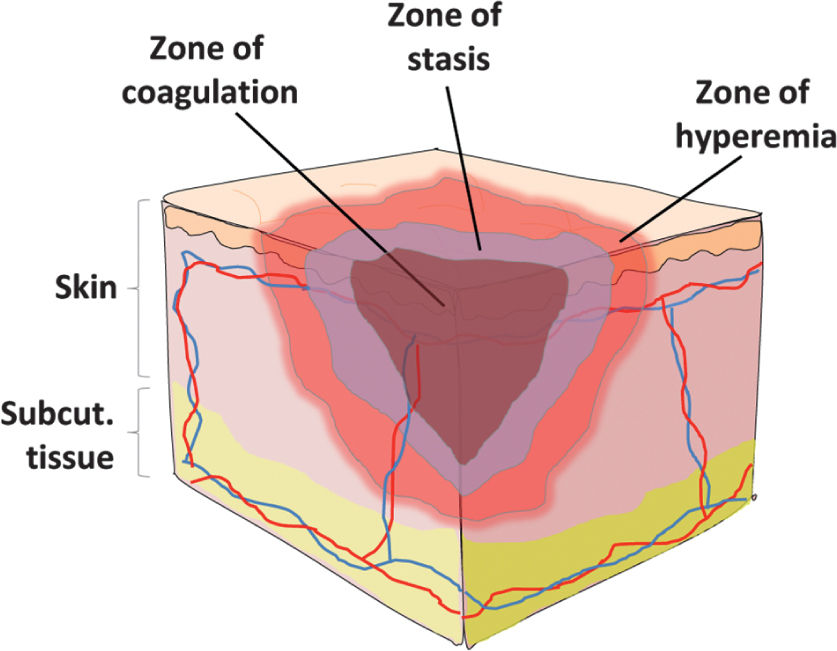
FIGURE 34.2 Zones of burn injury. The central zone of coagulation describes coagulation necrosis and irreversible full thickness burn injury. The zone of stasis describes an intermediate level of injury severity and depth that may be reversible. The zone of hyperemia describes increased vasodilation surrounding the burn area which should heal once the acute inflammatory phase subsides.
B. Arterial lines can be helpful given difficulty using cuff pressures in very edematous extremities.
1. The arterial line with central venous catheter can also be used to assess cardiac output when using the PiCCO system. This allows the provider to trend values, which may be more valuable than absolute numbers.
C. Pulmonary Artery Catheters. As in other areas of critical care, the PA catheter allows for goal-directed therapy. Studies have failed to show improvements in outcomes using PA catheters. They may be more useful in older patients with histories of cardiac disease.
V. MECHANICAL VENTILATION
Similarly mechanical ventilation can be employed with the following considerations:
A. Early endotracheal intubation is important in patients with inhalation injury or who have burns covering a lot of body surface areas. Airway protection is needed in patients requiring large-volume resuscitation. Other common indications for intubation include hypoxia and hypercarbia as well as inhalation injury.
B. Follow ARDSnet protocol recommendations. Low-volume protective lung ventilation: 4 to 6 mL/kg; plateau airway pressures should not exceed 30 cmH2O. Assess best PEEP trial to set PEEP, but a PEEP of at least 5 cmH2O should be used to avoid derecruitment. Consider esophageal manometry to aid in pleural pressure measurements if patient is obese. Recruitment maneuvers may be needed. Permissive hypercapnea is preferred over large tidal volumes. Limited studies have shown a potential role for high-frequency percussive ventilation in burn patients. Keep HOB 45° to decrease swelling and to prevent aspiration and ventilator-associated pneumonia in all ventilated patients. Daily mouth care with chlorhexidene should be used for ventilated patients.
VI. CONSIDERATIONS SURROUNDING RESUSCITATIVE STRATEGIES
Similarly, the following considerations surrounding resuscitative strategies should be employed and are temporally bound:
A. First 24 hours. Expect massive fluid shifts in both burned and nonburned tissues. Release of proinflammatory mediators such as TNF-α, histamine, and leukotrienes lead to increased microvascular permeability, edema, and shock. In general, resuscitation should be considered only in patients who have more than 30% total body surface area (TBSA) burns. It is important to get at least two large bore peripheral IVs. Starting point for fluids includes lactated ringers at 2 to 4 mL/kg × %TBSA burn with the first half given in the first 8 hours from injury (not arrival) and the second half given in the subsequent 16 hours. Children weighing less than 20 kg do not have large liver glycogen stores and should receive 0.45% saline with 5% dextrose at 3 to 4 mL/kg/h in addition to resuscitative fluid.
B. During resuscitation, it is imperative to follow the clinical response by monitoring: urine output, lactate, base deficit, mixed venous O2 (and central venous O2 if a PA line is in), central venous pressure, and cardiac output if using PiCCO or pulmonary artery catheter. It has been recommended to maintain urine output of about 0.5 mL/kg/h in adults and 0.5 to 1.0 mL/kg/h in kids; however, urine output can lag behind resuscitation and care must be taken not to overresuscitate. Studies do not necessarily demonstrate improved outcomes with either of these devices. PA catheters may lead to overresuscitation in healthy patients but are helpful in patients with cardiac diseases and those at risk of cardiac shock and congestive heart failure. It is important to assess for the presence of compartment syndrome of the extremities. This is done by clinical exam and by pulse examination. Monitoring for abdominal compartment syndrome is done by transducing bladder pressures and noting if there is increased difficulty ventilating and oxygenating, due to decreased venous return. Ocular compartment syndrome can be assessed using a tonometer.
C. Second 24 hours. All patients should receive crystalloid to maintain urine output and to maintain parameters of perfusion. Monitoring for adequacy of perfusion is done by measuring lactate, pulse volume variation, and cardiac outputs. Nutritional support should be started, usually by employing enteral nutrition within 24 to 48 hours. After 24 to 36 hours, intravenous fluids can be decreased by one-third, as long as the patient continues to produce adequate urine. Intravenous fluids can again be decreased by one-third for hours 36 to 48 (assuming urine output does not drop off). Colloids can be given after initial crystalloid resuscitation (5% albumin at 0.3–0.5 mL/kg/%TBSA over 24 hours) if blood pressures are not adequate.
D. After 48 hours, intravenous fluid should be administered to maintain urine output at 0.5 to 1 mL/kg body weight/h. Insensible losses and hyperthermia are associated with a hyperdynamic state. Daily weights can be helpful to determine insensible fluid loss or fluid retention.
E. Overresuscitation. If during the first 24 hours, resuscitation exceeds 6 mL/kg/%TBSA burn/24 h, the physician should reassess the clinical picture. An exuberant administration of intravenous fluid can lead to abdominal or extremity compartment syndrome, decreased chest wall compliance, and elevated peak airway pressures. Hourly neurovascular exams should be performed of all extremities at risk. Since patients are frequently intubated, physical exams can be limited. However, capillary refills can be assessed, as can pulse oximetry and compartment pressures with a Stryker needle or arterial line setup. If circumferential eschar is causing concern for compartment syndrome, extremity escharotomy should be made through the dermis down to the underlying adipose tissue but not through the muscular fascia (Fig. 34.3). Incisions should be made laterally and medially across affected extremities. Carpal tunnels may also need to be released (Fig. 34.4).
1. Chest compartment syndromes. The diagnosis is based on decreased chest wall compliance and increased peak airway pressures. Chest escharotomy should be performed along the anterior axillary line and connected at the infraclavicular and subcostal lines.
2. Abdominal compartment syndrome. The diagnosis is based on exam and bladder pressures >30 mmHg. Escharotomies laterally can also help prevent abdominal compartment syndrome. Once present, however, a bedside decompressive laparotomy may be needed, especially if high bladder pressures and high peak airway pressures remain after completion of the escharotomy.
3. Orbital compartment syndrome is relieved with lateral canthotomy.
VII. INHALATION INJURY AND CARBON MONOXIDE POISONING
A. Inhalation injury is divided into (1) upper airway (pharynx and trachea), (2) lower airway and parenchyma, and (3) toxicity due to toxic gases.
1. Upper airway. Swelling maximal at 12 to 24 hours. It can be diagnosed at the time of direct laryngoscopy. Upper airway has large absorptive capacity and prevents most burns to lower airway. Decision to intubate should be based on patient exam, pharyngeal swelling, carbonaceous sputum below the vocal cords, and mucosal swelling. Treatment of upper airway burns includes admission to hospital, humidified oxygen, general pulmonary toilet, and bronchodilators if indicated. Intubation is provided if indicated by experienced provider with access to fiberoptic technology and surgical intervention. If intubated, larger tube allows for better bronchoscopy and pulmonary toilet.
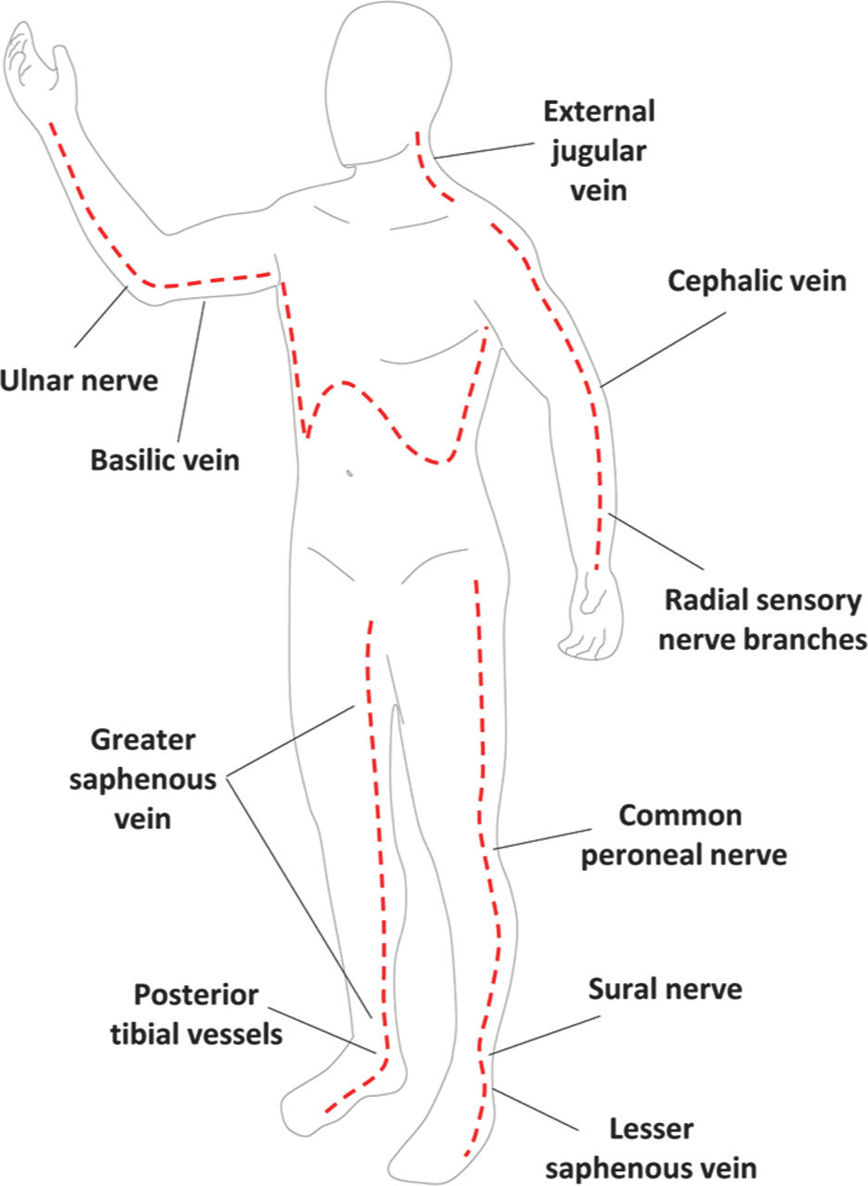
FIGURE 34.3 Schematic of burn escharotomy incisions. Dashed red lines depict full thickness incisions through burned skin down to subcutaneous fat. Labels point to key anatomic structures that should be preserved whenever possible when performing escharotomies.
2. Lower airway. Caused by smoke combustion products and inhaled steam. It results in loss of ciliary clearance of damaged mucosa and debris leading to a high incidence of pneumonia (50%).
VIII. SIGNIFICANT MORBIDITY AND MORTALITY ASSOCIATED WITH THERMAL BURNS WITH INHALATIONAL INJURIES
A. Mortality for burn patients on ventilator for more than 1 week is similar to that of ARDS. Burn patients are unique in their development of ALI and ARDS, as they suffer smoke inhalation, causing respiratory insufficiency, hypoxia by increased capillary permeability, ciliary dysfunction, and interstitial edema. Damaged necrotic respiratory mucosa will slough, causing bronchial plugging and atelectasis. Usually hypoxemia and ARDS do not develop until 4 to 8 days postburn. Whether smoke inhalation is a separate condition from ALI/ARDS is still debated. Treatment is supportive.
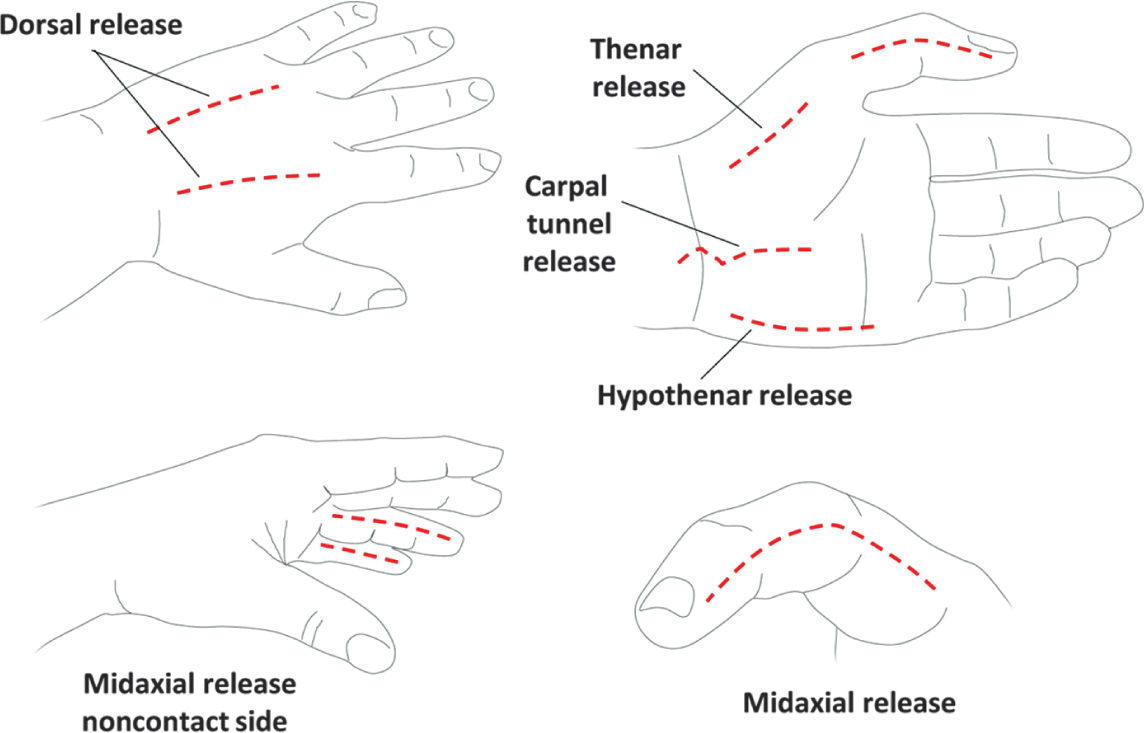
FIGURE 34.4 Schematic of burn escharotomy incisions for hand burns. Because these incisions are identical to those performed for hand compartment release, dissection should proceed bluntly through the muscular fascia if concern for hand compartment syndrome. Dorsal incisions should overlie the second and fourth metacarpals. Midaxial incisions should be on non-contact digit surfaces and avoid the volarly positioned neurovascular bundle.
B. Standard Diagnostics: bronchoscopy of upper and lower airway to look for soot tissue sloughing, mucosal necrosis, and carbonaceous material in the airway. Prophylactic antibiotics are not indicated. In all, 96% of patients will have positive bronchoscopy for inhalation injury if clinical triad of history of closed-space fire, increased serum carbon monoxide >10%, and the presence of carbonaceous sputum.
C. Treatment for Inhalational Injury. If inhalation injury is present, the patient should have airway assessment and be placed on supplemental oxygen. Aerosolized agents, including β-agonists, can improve pulmonary toilet in some patients. Do not continue these treatments if the patient does not respond. Necrotic endobronchial debris sloughing can make secretion clearance difficult. Therapeutic bronchoscopy may help clearance of debris, but data do not show improved outcomes with bronchoscopy performed to improve pulmonary toilet.
D. Pulmonary Infection. Occurs in 30% to 50% of patients. Patients with purulent sputum, fever, and impaired gas exchange should be treated for pneumonia. Chest radiographic findings and leukocytosis should make the provider suspicious that the patient has a chest infection. If the patient has pneumonia and been in the hospital for more than 48 hours, he or she should be treated for hospital-acquired pneumonia. Sputum should be sent for aerobic, anaerobic, and quantitative cultures. Empiric antibiotics including coverage of Pseudomonas should be started until cultures come back. Empiric treatment should cover MRSA, and thus vancomycin or linezolid are preferred. If vancomycin is used, it is important to check vancomycin levels to avoid renal toxicity.
E. Ventilator-Associated Pneumonia (VAP). Staphylococcus aureus and Streptococcus are common causes of early VAP. Pseudomonas aeruginosa is the most common cause of late VAP. The normal diagnosis of pneumonia (fever, purulent sputum, or leukocytosis) may not be helpful in burn patients, since almost all patients are febrile, tachypneic, and have elevated white blood cell counts. The best diagnostic test in this situation is probably a bronchoscopy-obtained bronchial alveolar lavage sample with quantification of the bacteria, >103 CFU. This level of bacteria is considered significant, as per ABA recommendations for VAP treatment. Antibiotic treatment duration for pan-sensitive organisms is 8 days. Duration of antibiotic treatment for multidrug-resistant organisms is 15 days. Providers should consider prophylactic antifungals (diflucan) if the patient has received prolonged broad-spectrum antibiotics.
F. Carbon Monoxide. Increased carbon monoxide is found commonly in fires from enclosed settings. Injury occurs from a combination of increased concentrations of carbon monoxide, the presence of both anoxia and hypotension. Carbon monoxide binds to heme-containing enzymes, such as hemoglobin, more avidly than oxygen. If carboxyhemoglobin is greater than 25%, patient should receive hyperbaric oxygen, if they are stable enough to tolerate movement to a chamber. Typical hyperbaric oxygen regimens include exposure to three atmospheres for 90 minutes with 10-minute breaks. Contraindications to HBO include unstable hemodynamics in patient with wheezing or auto-PEEP, high fevers, and a risk of seizures.
IX. METABOLIC RESPONSE AND NUTRITION
A. Metabolic rates in burn patients are significantly greater than other critically ill patients, leading to lean body mass wasting. Positive nitrogen balance is the key to prevent skeletal muscle breakdown.
B. General composition of the enteral feeding should include at least 60% calories from carbohydrates, but not exceeding 1,600 kcal/d, 12% to 15% lipids and essential fatty acids, and 20% to 25% protein. Failure to meet large energy and protein requirements can impair wound healing and alter organ function. Adequate nutrition is crucial and overfeeding must be avoided. Previously, a randomized, double-blind, prospective study demonstrated that aggressive high-calorie feeding with enteral and parenteral nutrition was associated with increased mortality. The daily caloric requirements should be calculated using the Curreri formula: [(25 kcal) (body wt kg)] + [(40) (%TBSA burn)] 1 to 2 g/kg/day of protein for synthetic needs of the patient. Burn patients should receive less percentage calorie requirements from fat than other ICU patients. Livers in burn patients have less VLDL, causing hepatic TG elevation. Increased fat consumption leads to increased complications including fatty liver, infection, hyperlipidemia, hypoxia, and mortality.
C. Micronutrients. There is decreased gastrointestinal absorption and increased urinary losses of micronutrients. Thus, burn patients can develop deficiencies in vitamin C, vitamin E, zinc, iron, and selenium. Burn patients have been shown to benefit from glutamine supplementation.
D. Acute response to thermal injury is biphasic, with the hypodynamic shock state at 24 to 72 hours and hyperdynamic catabolic state on the 5th postburn day. Patients have supraphysiologic cardiac outputs, elevated body temperatures, supranormal oxygen consumption, supranormal glucose consumption, altered glucose metabolism, and increased CO2 production due to accelerated tissue catabolism. This hyperdynamic catabolic state is thought to be caused by an excessive release of catabolic hormones, including catecholamines, glucagon, and cortisol. A shift from an anabolic–catabolic homeostasis to this hypercatabolic state leads to an increased need for energy, leading to skeletal muscle loss, which is broken down to maintain a sufficient supply of glucose and amino acids. The increased resting metabolic rate is directly related to the severity of the burn injury. A persistent hypermetabolic state is unsustainable. To decrease this hypermetabolic state, the provider should attempt to decrease the catabolic response by treating sepsis, performing early excision and skin grafting, and maintaining the core body temperature between 38 and 38.5°F. A nonselective β-blocker, such as propranolol, can also be used, as it inhibits the effect of catecholamines and slows muscle catabolism. Herndon et al. demonstrated that the usage of propranolol for the treatment of acute burns in the pediatric population improved net muscle synthesis and increased lean body mass.
E. Other medications to consider include pharmacological agents that convert catabolism to anabolism: oxandrolone (a testosterone analog given 0.1 mg/kg q12h) improves muscle synthetic activity, increases expression of muscle anabolic genes and increases net muscle protein synthesis. These effects improve lean body mass composition and have reduced weight loss. This also improves donor site wound healing and decreases the duration of the hospital stay. The provider needs to check liver function tests weekly due to the risk of transaminitis when receiving anabolic steroids. The practice has been to continue oxandrolone for 6 months. Human growth hormone and insulin-like growth factors have also been investigated, but the results of their administration have been mixed.
F. Glucose Control. Increase in hepatic gluconeogenesis and impaired insulin-mediated glucose transport into skeletal and cardiac muscle and adipose tissue occur regularly in burn patients. Hypermetabolism leads to hyperglycemia and insulin resistance. Data do not support strict glucose control (<110 mg/dL), but moderate blood glucose control is recommended (should be kept <180 mg/dL).
X. ELECTROLYTE ABNORMALITIES AND ACUTE RENAL FAILURE
A. The immediate effect of burn injury on kidney function occurs when there is hypovolemia (see above) and because of the release of cytokines.
B. A diuretic phase is seen at 48 to 72 hours after “third space” fluid is reabsorbed.
C. Hyponatremia is often seen due to large-volume resuscitation with hypotonic IV fluids. This will self-correct. Open burn wounds or use of topical silver nitrate can also cause hyponatremia. Be aware that renal hypoperfusion exacerbates hyponatremia by decreasing glomerular filtration.
D. Hypernatremia is due to insensible and evaporative water losses.
E. Providers should monitor patients for hyperkalemia and hypokalemia.
F. Hypophosphatemia can result from dilution or when a refeeding syndrome occurs.
G. Hypocalcemia can result from the use of silver nitrate.
H. Acute Kidney Injury. Risk factors for acute kidney injury include sepsis, TBSA burns, organ failure, and antibiotic administration. Physiologically in burns, there is a decrease in renal perfusion, glomerular filtration rate, and renal plasma flow. The renal medulla is the most sensitive to hypoxia, and renal tubular cells are the most vulnerable.
1. Presentation: oliguria and decreased creatinine clearance
2. Early renal failure: often results from hypovolemia-induced ischemic injury. Early fluid resuscitation is important to prevent this. Overresuscitation can cause abdominal compartment syndrome, which will also compromise renal perfusion. Renal failure can also be caused from rhabdomyolysis, most commonly seen in burns due to electrical injury.
3. Late renal failure: occurs after the fifth postburn day. Can be caused by sepsis. And nephrotoxic antibiotics. Renal replacement therapy (CRRT) is thought to improve outcomes in patients with burns and AKI, but studies are still underway.
4. Pharmacologic treatments: Dopamine has not been shown to improve outcomes. However, the dopamine-1 receptor agonist, fenoldapam, at low doses has showed promise in studies by increasing urine output and decreasing serum creatinine. Lasix and mannitol will improve urine output, but have not been shown to improve outcomes in burn patients.
5. Initial care of ARF patients should focus on reversing the underlying cause, as well as correcting any fluid and electrolyte imbalances. Ensure adequate volume status, avoid nephrotoxins, and dose medications appropriately. A high aldosterone response often necessitates potassium supplementation.
XI. CHEMICAL BURNS
A. General Approach to Chemical Burn Treatment. Protect yourself with personal protective equipment—always consider that the chemicals are still present and must be neutralized or temporized. With few exceptions (see below), all chemical burns should be copiously irrigated with water. Water dilutes, but does not neutralize, the chemical and cools the burning area. Neutralization of chemical burns is generally contraindicated because neutralization may induce a hyperthermic reaction. Water irrigation is contraindicated or ineffective in several scenarios, including the following:
1. Elemental sodium, potassium, and lithium: these both may precipitate an explosion.
2. Dry lime, which should be brushed off and, not irrigated.
3. Phenol, which is water insoluble and should be wiped from the skin with polyethylene glycol-soaked sponges.
B. Types of Chemical Burns
1. Alkali: causes liquefaction necrosis and protein denaturation. Found in oven and drain cleaning products, fertilizers, and industrial cleaners. Alkali injuries extend deeper into tissues until the source is removed or diluted and are especially disruptive to the eye.
2. Acids: damage tissue via coagulation necrosis and protein precipitation
3. Organic compounds (phenol and petroleum): cause damage via cutaneous damage due to fat solvent action (cell membrane solvent action) and systematic absorption with toxic effects on the liver and kidneys. These can also cause significant erythema in the surrounding areas that may be mistaken for cellulitis.
C. Specific Types of Chemical Burns
1. Hydrofluoric acid (HF): potent and corrosive acid used as a rust remover, in glass etching, and to clean semiconductors. Although a weak acid, the fluoride ion is toxic. It causes severe pain and local necrosis. Treatment is copious water irrigation. Fluoride ion is neutralized with topical calcium gel (one amp calcium gluconate in 100 g lubricating jelly). If symptoms persist, intra-arterial calcium infusion (10 mL calcium gluconate diluted in 80 mL of saline, infused over 4 hours) and/or subeschar injection of dilute (10%) calcium gluconate solution are recommended. Fluoride ion binds free serum calcium; make sure to check the serum calcium and replace with IV calcium as needed.
2. Phenol: commonly used in disinfectants and chemical solvents. Phenoal is an acidic alcohol with poor water solubility and causes protein disruption and denaturation, resulting in coagulation necrosis. It is responsible for cardiac arrhythmia and liver toxicity: cardiac and liver function should be monitored. It is cleared by the kidneys. Phenol causes demyelination and has a local anesthetic effect, and thus pain is not a reliable indicator of injury. Recommended treatment is copious water irrigation and cleansing with 30% polyethylene glycol or ethyl alcohol. EKG monitoring is required.
3. Tar: used in the paving and roofing industry. It can be heated to 260°C (~500°F) prior to application and causes thermal injury; tar solidifies as it cools and will become enmeshed with hair and skin. It should be cooled with copious water irrigation to stop the burning process. Tar removers promote micelle formation to break the tar–skin bond. Sterile surfactant mixture (De-Solv-it or Shur-Clens) allows tar to be wiped away in real time. Applying wet dressings using polysorbate (Tween 80) or Neomycin cream for 6 hours prior to tar removal can also be effective.
4. White phosphorus: used to manufacture military explosives, fireworks, and methamphetamine. Obvious particles should be brushed off. Skin should be irrigated with a 1% to 3% copper sulfate solution. Copper sulfate stains the particles black for identification. Copper sulfate will also prevent ignition when particles are submerged in water. After copper sulfate irrigation, the exposed area should be placed in a water bath and the white phosphorous should be removed.
5. Anhydrous ammonia: alkali used in fertilizer. Skin exposure is treated with irrigation and local wound care. Exposure is associated with rapid airway edema, pulmonary edema, and pneumonia. Always consider early intubation for airway protection.
6. Methamphetamine: causes tachycardia (greater than expected with a similar size burn), hyperthermia, agitation, and paranoia
D. Injury to Eyes: Treat with prolonged irrigation with Morgan lenses. Eyelids may need to be forced open due to edema or spasm. Utilize a topical ophthalmic analgesic and consult an ophthalmologist.
XII. ELECTRICAL INJURY
A. Unlike other burns, TBSA is not necessarily associated with prognosis and TBSA does not quantify damage to deeper tissues.
B. Mechanism of injury
1. Thermal: can generate temperatures above 100°C
2. Electroporation: electrical force drives water into lipid membrane, causing cell rupture
3. Difficult to determine type and severity of damage between entrance and exit
4. Tissue resistance in decreasing order = bone, fat, tendon, skin, muscle, vessel, nerve
5. Bone heats to a high temperature and burns surrounding structures.
C. Injury Severity. Determined by voltage, current type, and resistance. High-voltage burns are considered to be those involving >1,000 volts. Alternating current causes tetanic muscle contraction and the “no let-go” phenomenon. This occurs due to simultaneous contraction of (stronger) forearm flexors and (weaker) forearm extensors.
D. Current flow through tissue can cause burns at entrance/exit wounds and burns to deep tissue. A current will preferentially travel along low-resistance pathways. Current will pass through soft tissue, contact high-resistance bone, and travel along bone until it exits to the ground. Vascular injury to nutrient arteries through damage to their intima and media leads to thrombosis.
E. Cardiac Effects. Arrhythmia. Recommend an EKG monitor for at least 24 hours, watching for coronary artery spasm, myocardial injury, and infarction.
F. GI Effects. Injury to solid organs, acute bowel perforation, and gallstones after myoglobinuria
G. Initial monitoring:
1. Airway maintenance: C-collar until c-spine cleared
2. Breathing and ventilation—100% oxygen
3. Circulation and cardiac status: Cardiac monitor, two large-bore IVs, assess peripheral perfusion, EKG
4. 24-hour monitoring indicated if any of these is present: ectopy or dysrhythmia, loss of consciousness, cardiac arrest, or abnormal rate or rhythm
5. Disability, neurological deficit, and gross deformity: Assess level of consciousness, note any neurological deficit, and note any gross deformity.
6. Exposure and environmental control: Stop the burning process, then remove clothes and avoid hypothermia.
7. Perform renal function analysis and urine myoglobin.
H. Fluid Resuscitation. TBSA provides inadequate estimation of electrical burn severity. Unlike thermal injury, electrical injury often occurs deep to the skin and is not visible. Standard fluid resuscitation models (Parkland) may underestimate fluid resuscitation needs. If no urine pigmentation is present, the minimum acceptable urine output is 0.5 mL/kg/h. Pigmented urine can be caused from myoglobin (secondary to rhabdomyolysis) and/or free hemoglobin (from damaged RBCs). For myoglobinuria, the urine dipstick will be positive for blood and microscopy will not demonstrate RBCs. The goal for urine outputs when rhabdomyolysis and myoglobinuria has occurred is 2 mL/kg/h or about 75 to 100 cc/h. Insufficient volume resuscitation can predispose to myoglobin-induced acute tubular necrosis. In addition to adequate fluid resuscitation, myoglobin excretion can be enhanced using mannitol (12.5 g/h osmotic diuresis) and/or urine alkalinization with 50 mEq/L of bicarbonate. Follow urine myoglobin levels every 6 hours until a downward trend is seen.
I. Compartment Syndrome can occur after high-voltage injury to an extremity. Current travels along bone, which has high resistance. The bone serves as a conductor and “cooks” adjacent tissue from deep to superficial. In the upper extremity, FDP and FPL will be most severely affected (closest to bone). Overaggressive fluid resuscitation can worsen tissue edema, resulting in increased tissue pressures and exacerbating raised compartment pressures, typically occurs within 48 hours of injury. Clinical concern for raised compartment pressures mandates an evaluation of compartment pressures or a trip to the operating room. The 6 “P” signs/symptoms include pain out of proportion, paresthesia, pallor, paralysis, pulselessness, and poikilothermia. Elevated compartment pressures can be used as an adjunct to clinical diagnosis or for cases where the patient is unable to participate in clinical exam. Absolute pressure ≥30 mmHg or elevated pressure within 20 mmHg of the diastolic blood pressure is also diagnostic of compartment syndrome. Forearm compartment syndrome is managed via surgical release of volar and extensor compartments and the mobile wad. In the hand, release carpal tunnel, Guyon’s canal, and the nine compartments of the hand. Lower extremity compartment syndrome is managed with fasciotomies of the anterior, lateral, superficial posterior, and deep posterior compartments.
XIII. BURN WOUND MANAGEMENT
A. Topical Antimicrobials
1. Silver sulfadiazine. Broad-spectrum antimicrobial activity due to silver ion. Soothing sensation that does not cause significant metabolic or electrolyte complications. Does not penetrate eschar. Can cause neutropenia but this is often self-limited. Assess if patient has sulfa allergy.
2. Sulfamylon (mafenide acetate). Penetrates eschar and is useful on ears to prevent suppurative chondritis. Can be used as a cream or as a soak (2.5% or 5%). It does not stop fungi, which silver nitrate does. Can also cause metabolic acidosis due to its inhibition of carbonic anhydrase. Can be painful on partial-thickness burns.
3. Silver nitrate. Offers broad-spectrum antimicrobial coverage against gram-positive and gram-negative bacteria and fungal coverage. Delivered as an aqueous 0.5% solution every 4 hours to keep dressing moist and prevent precipitation of the silver nitrate. Side effects include staining skin (and anything it contacts) black, hyponatremia, and hypomagnesemia. Rare instances of methemoglobinemia necessitating methylene blue treatment.
4. Mupirocin: Effective against MRSA and can be used in addition to other topicals to expand coverage
XIV. COLD INJURY
A. Frostbite occurs in response to a slow rate of cooling with ice crystal formation in tissue when tissue temperature <28°F.
B. Concentrated solutes draw fluid out of cells and ice crystals subsequently cause cell membrane puncture.
C. Intravascular ice crystals cause direct vascular damage and indirect vascular sludging.
D. With rewarming, tissue thaws from the blood vessels outward.
E. Freeze-induced endothelial damage allows capillary leaking to occur. This allows extravasation of PMNs and mast cells, causing inflammation, edema, microvascular stasis, and occlusion.
F. Blisters will form at 6 to 24 hours when extravasated fluid collects beneath the detached epidermal sheet. If the dermal vascular plexus is disrupted, hemorrhagic blisters will be present.
G. Stages of Frostbite
1. 1st degree: hyperemia, intact sensation, no blisters on rewarming, no tissue loss expected
2. 2nd degree: blisters containing clear or milky fluid, local edema, no tissue loss expected
3. 3rd degree: hemorrhagic blisters, edematous tissue, shooting or throbbing pain, and likely tissue loss
4. 4th degree: mottled or cyanotic skin, hemorrhagic blisters, and frozen deeper structures. Mummification occurs over several weeks.
H. Treatment
Multiple freeze–thaw cycles causes multiplicative, not additive, damage to the affected tissues. Intact blisters should be left alone. Debride ruptured blisters and apply bacitracin ointment or silvadene. Beware of the “afterdrop” phenomenon during rewarming, where central rewarming results in peripheral vasodilation. This returns cold blood from the extremities to central circulation and can result in systemic hypothermia. Rapid rewarming of affected area in 104° to 108°F water bath, not radiant heat. Additional strategies include ibuprofen 400 mg PO q12h; penicillin 600 mg q6 × 48 to 72 hours; elevation of limb with splinting to decrease movement; no smoking, caffeine, or chocolate; and tetanus prophylaxis. Three-phase bone scan may identify “at risk” tissue.
I. Acute Interventions after Warming
1. For stable patients with severe frostbite, rapid extrication to a center with interventional radiology capabilities within 12 hours is indicated. Arterial catheterization can identify and treat vasospasm and microvascular thrombosis with tPA or heparin. Catheter-directed tPA should be given for 24 hours and then the patient should be placed on a heparin gtt.
2. Reversal of local microvascular thrombosis may restore perfusion before irreversible necrosis and ischemia occur.
3. Several studies have shown significant decrease in amputation rates and tissue loss with this aggressive protocol.
4. Early regional sympathectomy of an affected extremity is controversial.
XV. STEVENS JOHNSON SYNDROME (SJS) AND TOXIC EPIDERMAL NECROLYSIS SYNDROME (TENS)
A. Both SJS and TENS have widespread necrosis of the superficial portion of the epidermis.
B. SJS/TENS is commonly associated with sulfonamides, trimethoprim-sulfamethoxazole, oxicam NSAIDs, chlormezanone, and carbamazepine. A single offending drug is identified in less than 50% of cases. Antibiotic-associated SJS/TENS presents ~7 days after drug is first taken. Anticonvulsant-associated SJS/TENS can present up to 2 months after drug is first taken.
C. TENS can also be caused by staphylococcal infections in immunocompromised patients.
D. SJS—total involvement less than 10% TBSA. Widespread erythematous or purpuric macules or flat atypical targets are present.
E. Overlap SJS-TENS—total cutaneous involvement of 10% to 30%. Widespread purpuric macules or flat atypical targets are present.
F. TENS with spots—total cutaneous involvement of greater than 30% TBSA. Widespread purpuric macules or flat atypical targets are present.
G. TENS without spots—total cutaneous involvement greater than 10% TBSA. Large epidermal sheets present. No purpuric macules or targets.
H. Initial symptoms can be a 2-to-3-day prodrome of nonspecific findings such as fevers, headaches, and chills. Symptoms of mucosal irritation like conjunctivitis, dysuria, and/or dysphagia may be present. These symptoms are followed by mucosal and cutaneous lesions.
I. Mucosal irritation typically occurs at two or more sites. Involved sites may include vaginal, urinary, respiratory, gastrointestinal, oral, and/or conjunctival.
J. Skin lesions are diffusely present. Lesions are typically erythematous macules with purple, possibly necrotic, centers. Nikolsky’s sign is typically positive (rubbing the skin causes exfoliation of outermost layers and/or a new blister to form).
K. Differential diagnosis of acute, diffuse blistering includes staphylococcal-scalded skin syndrome, pemphigus vulgaris, pemphigus folaceus, paraneoplastic pemphigus, bullous pemphigoid, acute graft-versus-host disease, and linear IgA dermatosis.
L. Diagnosis of SJS/TENS is largely clinical and can be confirmed by both skin biopsy and histology.
M. Treatment. Discontinue all potentially offending drugs. Transfer to a burn ICU for fluid/electrolyte monitoring, dressing changes, and temperature regulation is recommended. Debride flaccid bullae. Administer initial wound care with dressing changes until extent of skin loss is known. All necrotic epidermis should be debrided to reduce bacterial growth, and area should be covered with biological or synthetic dressings. Recommend human allograft and porcine xenograft placement to the denuded areas to reduce pain, decrease fluid loss, and promote healing. Empiric systemic antibiotics have been associated with increased mortality and are not indicated. Topical antimicrobials such as silver sulfadiazine, silver nitrate, and polymyxin-bacitracin can help prevent infection; however, silver sulfadiazine should be avoided due to its sulfonamide component. Consider hemodialysis to remove potentially offending drugs with long half-lives. Corticosteroid and cyclosporine A therapy has not been supported by current data and is not recommended. Early ophthalmology consultation is recommended. More than 50% of SJS/TENS patients can develop symblepharon or entropion. Treatment can involve other consultant services (pulmonary, urology, gynecology, gastroenterology) as needed. Administration of steroids and IVIG is controversial. TENS is known to overexpress FAS, which promotes apoptosis of keratinocytes by binding to the FAS/CD95 receptor. IVIG blocks the CD95 receptor and has been efficacious in small series of TENS patients.
XVI. PRESSURE ULCERS
A. Definition. Necrosis and tissue breakdown from pressure.
B. Etiology. Tissue ischemia from external pressures exceeding the closing pressure of nutrient capillaries (32 mmHg) for a prolonged duration. Pressures exceeding 70 mmHg for 2 hours cause ischemia. Shear forces cause vessel stretch, leading to thrombosis. Friction causes epidermal injury (e.g., during transfers). Excess moisture causes skin maceration and increased pressure sore risk. Ischemia–reperfusion cycle plays a role. Decreased autonomic control lead to spasm, loss of bladder, and bowel control, and excessive sweating lead to increased moisture. Advanced age causes decreased skin tensile strength. Malnutrition depletes patient of protein and vitamins needed for wound healing. Sensory loss causes inability to experience discomfort or tissue ischemia.
C. Epidemiology/Natural History. Prevalence is 15% in general acute care setting. Incidence is 0.5% to 38% in general care settings. More than 60% of patients with pressure sores are >70 years of age. Additional risk factors include cerebrovascular disease, previous pressure sore, immobility (debilitated or paralyzed), malnutrition, end-stage renal disease, and diabetes mellitus. Chronic polymicrobial colonization is defined as count >1 × 105 per mm3 tissue. Staphylococcus aureus and Streptococcus are the most common bacteria. Chronic wound has possibility of malignant degeneration (Marjolin’s ulcer).
D. Prevention: Avoid moisture through bladder/bowel hygiene, avoiding soilage. Control of spasticity facilitates proper positioning, and baclofen or diazepam treatment can also help with spasticity. Proper pressure distribution using air fluidized, low air-loss, and alternating air cell mattresses. Pressure relief protocols recommend repositioning recumbent patients every 2 hours.
E. Diagnosis/Workup. Perform laboratory studies and imaging, complete blood cell count (CBC) with differential, glucose/hemoglobin A1c, albumin/prealbumin, erythrocyte sedimentation rate (ESR)/C-reactive, protein (CRP), and MRI.
1. Stages defined by National Pressure Ulcer Advisory Board (NPUAP) (Fig. 34.5):
a. Stage I: nonblanchable erythema present for >1 hour after pressure relief. Skin intact
b. Stage II: partial-thickness skin loss
c. Stage III: full-thickness skin loss into subcutaneous tissue but not through fascia
d. Stage IV: through fascia into muscle, bone, tendon, or joint
e. Note: If eschar is present, wound cannot be staged until fully debrided.
2. Muscle is more susceptible to ischemia than is skin. Muscle necrosis may have occurred with skin erythema as the only sign.
3. Nutritional status: Serum albumin <3.5 mg/dL may be a risk factor for developing pressure sores.
4. Osteomyelitis: Presence of bone infection must be determined. Initial studies include ESR, CRB, CBC, with ESR >100 usually indicative of osteomyelitis. MRI can identify osteomyelitis and extent of disease. T2-weighted images will show enhancement in region of osteomyelitis. Bone biopsy remains the gold standard for diagnosis: Send one sample to pathology and one sample for quantitative microbiology.
5. Identify contractures and spasticity in paraplegic and quadriplegic patients.
6. Assess bowel/bladder routine and continence.
7. Assess motivation and support structure including adherence to pressure relief protocols, adherence to wound care routines, maintenance of adequate nutrition, and participation in risk factor modification (e.g., smoking cessation).
F. Treatment Overview. Excise and treat infection and avoid new pressure sores. Surgical closure is not attainable in all patients (e.g., poor surgical candidates, nonoptimal social circumstances). Debridement of nonviable tissues is recommended. Provide wound care with dressing if wound is not ready to be closed. Continue with antibiotics for 6 weeks if osteomyelitis is present. Primary surgical closure is not ideal as maximal pressure point is directly under incision. Local rotational or advancement flaps using skin and/or fascia offer more robust coverage. Recurrence of ulcers is the rule, not the exception.
G. Wound Dressings
1. General: Achieve warm, moist, and clean environment for wound healing; desiccated wound needs hydration, and wound with excess drainage needs an absorbent. A wound with necrosis needs debridement, and infected wounds need antimicrobial dressings. Wet-to-moist dressing is recommended for debrided, clean-looking wound with normal saline and mesh gauze. In clean wounds, this prevents desiccation for optimal fibroblast and keratinocyte development and epithelial migration.
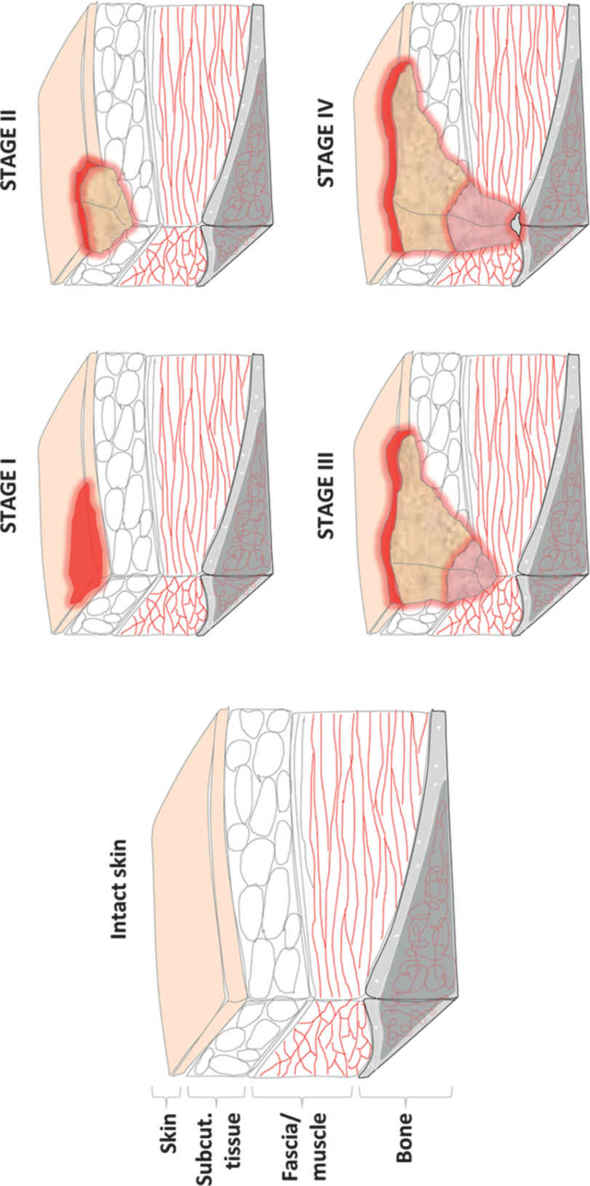
FIGURE 34.5 Schematic of stages of pressure ulcer formation. Stage I involves superficial injury with non-blanching erythematous changes but intact skin. Stage II describes partial thickness skin injury. Stage III ulcers have full thickness skin loss with exposed subcutaneous tissue. Stage IV ulcers extend through muscular fascia into muscle, tendon, or bone.

Full access? Get Clinical Tree








