Schematic drawing of our three body fluid compartments.
The sizes of these body fluid volumes have been measured under steady state conditions by the use of tracer methods. In an adult weighing 75 kg, they average 3 liters for the plasma, 12 liters for the interstitial fluid, and 30 liters for the ICF volume. Hence, the sum of the plasma and interstitial fluid volumes (the ECF volume) amounts to 15 liters, or 20% of the body weight.
Measures and estimates of body fluid volumes are of interest to basic research, while clinical guidance for fluid therapy is mostly guided by hemodynamic measurements, sometimes combined with fluid bolus infusions.
Tracers
Substances known to distribute solely within one body fluid compartment can be injected and the size of the compartment can be calculated by means of dilution of the substance.
The substance is then used as a tracer. The basic equation for such calculations is:

Examples of such tracers include bromide and iohexol for measurements of the ECF volume. Radioiodated albumin and indocyanine green (ICG) are used for measurement of the plasma volume.
Bromide has a very slow turnover, which means that the measurement might be problematic to repeat several times. Iohexol has a shorter half-life, only 100 min, but this also implies that an estimation of the ECF volume has to be based on several plasma samples to account for the elimination during the period of mixing. Sampling cannot start within 30–40 min because the kinetics shows a clear distribution phase.[1]
The total body water (sum of ECF and ICF) can be measured with water isotopes, which include tritium (radioactive) and deuterium (not radioactive). One problem is that even distribution of these molecules in the body requires about 3 hours to be completed. An alternative approach is to use ethanol, but the relatively short half-life requires frequent sampling in blood or in the expired air.[2]
The plasma volume has frequently been measured by radioactive iodated albumin. After 10 min of distribution of the injected substance, 3–4 samples are taken every 10 min to account for the exponential elimination. As the time window for the measurement is 30 to 40 min, the plasma volume needs to be in a reasonably steady state during that time to yield a correct result.
Plasma tracer results slightly overestimate the plasma volume. Therefore, the result should be multiplied by 0.91 (the “hematocrit factor”).
Evans blue is a dye that has been used as a substitute for the albumin method because the measurement does not involve exposure to radioactivity. The dye binds to albumin, just like iodine, and the concentration is measured by light absorption.
There are several possibilities for labeling erythrocytes with radioactive tracers to calculate red cell mass. Such tracers include chromium and technetium.
Carbon monoxide binds to hemoglobin and can also be used to measure red cell mass. Drawbacks are mainly safety issues, as carbon monoxide is toxic.
The size of the interstitial fluid space and the ICF volume cannot be measured by tracers. They have to be inferred as the difference between body fluid spaces that can be measured, i.e. the plasma volume, the ECF volume, and the total body water.
These conventional tracer methods of measuring body fluid volumes have limited application in perioperative medicine. A comparison with standard values would indicate whether the patient is dehydrated or hyperhydrated before or after an operation. However, conventional tracers cannot be used to reflect what happens during surgery because the situation is too unstable. During the mixing period there must be steady state with regard to fluid shifts, a situation that is hardly ever met in the operating room. It is unlikely that the results can be accurate in the early postoperative period. The only possible exception to this rule could be the ICG tracer because steady state is then needed only for a few minutes.
If applied during steady state conditions, the tracer methods have an accuracy of between 1% (total body water) and 10% (radioiodinated albumin).
A common way to determine the volume effect of an infusion fluid in the experimental setting is to measure the plasma volume at baseline and then again after an infusion fluid has been administered. With crystalloid fluid there is no meaningful way of measuring the volume effect with a tracer until equilibration with the interstitial fluid has occurred, because the fluid shifts are so fast. There is equilibrium 30 min after ending an infusion.
Indocyanine green
Indocyanine green (ICG) is a dye that binds to plasma albumin. The half-life is only 3 min, owing to rapid uptake by the liver. The rate of elimination is directly dependent on the liver blood flow. Therefore, ICG can be used to measure both liver blood flow (elimination slope) and plasma volume. Attempts have also been made to measure cardiac output with ICG.
To measure the plasma volume, steady state with regard to the fluid balance is required for only a few minutes.[3] Therefore, the ICG method can be applied during surgery.[4] The ICG concentration can be measured both in the blood and by pulse oximetry (pulse dye densitometry).[5] The precision is claimed to be a few percent.
Drawbacks include that the circulation time (about 1 min in normal humans) becomes crucial. ICG is best injected via a central venous catheter, and backward extrapolation should be made to 1 min and not to time zero, as elimination begins only after the tracer has reached the liver. Polidori and Rowley [6] have shown that extrapolation to time zero results in an underestimation of the true plasma volume in the normal human (Figure 7.2). In a cohort of patients they found the average error to be 25%. When comparing the plasma volume before and after volume expansion, the calculations will further be dependent on a constant relationship between changes in the liver blood flow and the time required for transport of the ICG to the liver.
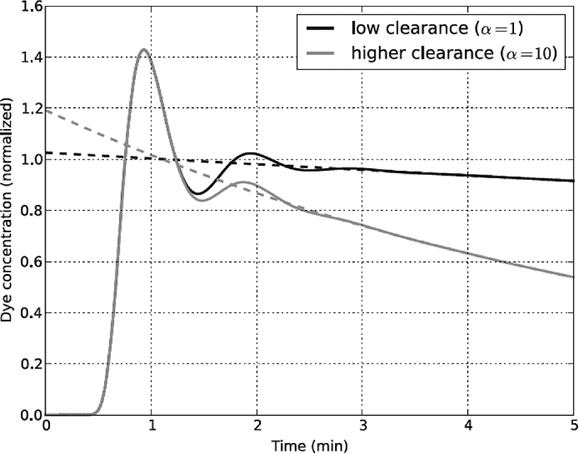
Why errors arise when back-extrapolation of the concentration–time curve of indocyanine green (ICG) overlooks the 1-min transit time for the dye to travel from the site of injection (time zero) to reach the site of elimination in the liver. α is the slope of the line.
Double-tracer techniques
Measurement of the blood volume (BV) by a double-tracer technique means that the erythrocyte and plasma volumes are determined by separate tracer methods and that the BV is the sum of them. Various combinations of tracers can be used, of which tagging of the erythrocytes with chromium and determination of the plasma volume with radioactive albumin or ICG are the most common.
A problem with all double-tracer techniques is that the hematocrit in sampled peripheral blood is higher than the hematocrit deduced from direct measurement of the plasma and erythrocyte volumes. To account for this discrepancy, a correction factor (“hematocrit factor”) of 0.91 has been introduced. This factor appears to be quite stable under various physiological circumstances [7] but has been given a number of different interpretations over the years.
In the 1950s and 1960s it was believed that the hematocrit in large vessels simply was higher than in the body as a whole. The hematocrit is lower in the capillaries, but the BV residing in them (5% of total) is too small to explain the hematocrit factor. A simplistic explanation is that that albumin but not the erythrocytes easily enters the liver sinusoids and some other perivascular spaces, which slightly exaggerates the plasma volume when measured by plasma-bound tracers.
A recent view was that only ICG but not erythrocytes and albumin penetrates the non-circulating plasma bound in the endothelial surface layer.[4] This is not a full explanation either, because the hematocrit factor is present also when measuring the plasma volume with the radioactive albumin method, where the tracer (albumin) is excluded from the endothelial surface.
Bioimpedance
In bioimpedance (BIA) measurements, a series of weak electrical currents of different frequency are passed through the body, typically running from the arm to the foot.[8] Use of the method for measuring body fluid volumes is based on the fact that currents have more difficulty passing through large amounts of water than small. An estimate of both the ICF and ECF volumes can be made as various frequencies pass through and outside cells with varying ease.
Bioimpedance is often applied while the patient is in bed before and after surgery, but rarely perioperatively owing to the risk of mechanical and electrical interference. In the author’s experience, BIA can provide useful data only for groups and has a place mainly as an adjunct to more precise methods.
Anthropometry
Empirical relationships may be used to estimate the size of the body fluid compartments at baseline.
The simplest of these states that the plasma volume corresponds to 4.5% of the body weight, the BV is 7% of the body weight, and the ECF volume makes up 20% of the body weight.[1] Total body water represents 50% of the body weight in the adult female. The percentage is 60% in young adult men and 50% in older men. Children have a higher percentage. Such rules are simple but useful for the clinician to remember.
More precise information can be obtained by regression equations. These are typically based on tracer measurements of body fluid volumes performed in a large number of humans, and usually employ the sex, body weight, and height of the subject as predictors. Below is an example of such equations for estimation of the BV in women and men.[9]
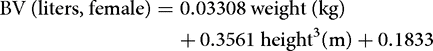
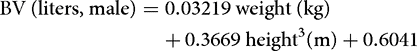
Sodium method
The distribution of infused fluid between the ICF and ECF may be estimated based on the use of serum sodium (SNa) as the marker. Sodium is then used as an endogenous tracer.
If all infused fluid and sodium, as well as all voided amounts, are known, the change in ICF volume can be estimated based on the assumption that sodium is evenly distributed in the ECF volume, which makes up 20% of the body weight. From time 0 to time t, we get:

This mass balance equation has mostly been used to calculate the intracellular distribution of electrolyte-free irrigating fluids,[10] but it can be used to estimate the intracellular distribution of any infusion fluid.[11] For example, the sodium method was used to demonstrate that acetated Ringer’s solution does not expand the ICF volume in volunteers, despite the fact that the fluid is slightly hypo-osmolar; the reason is that the urine excreted during the first 30 min after the infusions contained much lower concentrations of sodium than the infused fluid, while there was only a marginal concomitant increase in SNa.[12]
Translocation of fluid from the ICF space when infusing hypertonic saline can also be estimated without measuring SNa. This calculation is then based on the osmolar balance between the ECF and ICF spaces, of which one space gradually becomes expanded and the other concentrated as more fluid is infused.[13]
Central blood volume
Measurement of the intrathoracic or central BV is possible with several modern hemodynamic monitoring systems, such as the PiCCO and LiDCO. These apparatuses are used mainly to measure central flow rates and blood pressures, while the central BV is provided as an adjunct output. A central venous pressure line or arterial catheterization is needed to obtain the data on body fluid volumes.
Measurement of the central BV is of interest because it is more clearly related to hypovolemia-related physiological responses than to total circulating BV. However, little has been published on intrathoracic BV in connection with infusion fluids.
Fluid “efficiency”
The volume effect of an infusion fluid implies how much of the infused volume is retained in the bloodstream and expands the BV. The strength and the duration of BV expansion are the properties that represent the efficiency of the fluid. One may also talk about potency when comparing the efficiency of various fluids.
For this purpose, physiological endpoints may be used. For example, a certain amount of blood can be withdrawn and the amount of fluid then required to restore baseline physiological parameters (such as cardiac output) can be taken to represent the “efficiency”.[14]
Tracer methods (mostly radioiodinated albumin) have also been used to determine the efficiency of infusion fluids. This implies that the tracer method is applied twice and that the result before and after the infusion is compared. Assessment of colloid fluids is likely to work well by this approach. Studying the volume effect of crystalloids is more difficult because changes are more rapid; there is both a distribution phase, which cannot be covered by exogenous tracers, and, at least in conscious humans, a fairly rapid elimination. Most data relying on tracers refer to the period when distribution has already been completed and voiding has eliminated more than a negligible fraction of the infused crystalloid fluid volume.
Blood hemoglobin
A third and more simplistic approach to quantify the volume effect of an infusion fluid is to measure the blood hemoglobin (Hb) concentration before and after the infusion. Hemoglobin can be used as an endogenous tracer as it remains completely in the bloodstream, and it is reasonable to assume that dilution of its concentration is due to the infused fluid volume.
The use of Hb to calculate the efficiency of an infusion fluid is a frequently misunderstood method. Hemodilution is not a measure of the BV. In fact, Hb changes will be the same regardless of whether the BV is 1, 2, or 5 liters, as long as the number of Hb molecules within that volume remains unchanged. Hb changes are rather the inverse of the water concentration and indicate the volume amount of the infused water that is easily exchangeable with the water in the sampled (usually venous) blood. The water concentration of whole blood is about 80% and almost all the rest is Hb (about 1 kg); electrolytes and plasma proteins constitute only a few percent of the total. The water concentration is always increased when a plasma volume expander is infused because the main constituent of an infused fluid is water. The dilution of Hb is a much easier way to indicate the increase in water concentration than to desiccate blood samples to determine the water concentration, but the two methods yield the same result.[15]
The potential error that can arise is when the hemodilution is interpreted in terms of a change in BV and the hemodilution is then multiplied by the BV at baseline. Radio-albumin, Evans blue, and carbon monoxide may be used to measure BV before an infusion is provided. More frequently, one assumes the BV before the infusion based on some anthropometric equation.
The Hb concentration is measured before (Hb) and after (Hb(t)) the infusion:[16]
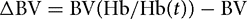
The amount of fluid retained in the blood is then given by:
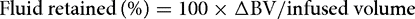
The fraction of fluid retained over time is the efficiency.
If the urinary output is known, the difference between the infused volume and the sum of the urine and blood volumes represents the change in interstitial fluid volume.[17]
Accounting for blood loss
The Hb dilution concept can be developed to account for blood loss, if known. This is (usually) necessary when calculating fluid shifts based on Hb during surgery. One then calculates the total Hb mass and subsequently subtracts all losses (or adds transfused erythrocytes):
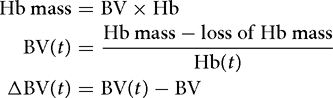
These simple equations can be entered into a pocket calculator and may be helpful for the clinician when assessing whether a patient is hypervolemic or hypo-volemic. The calculations can be applied repeatedly during surgery without loss of accuracy.[18]
These basic relationships shown above can, in turn, be further elaborated upon to quantify the efficiency of infusion fluids during ongoing surgery. An approach of that kind uses a multiple regression equation to separate the effects of various factors that influence the BV.

Full access? Get Clinical Tree




