Chapter 88 Blood transfusion
Blood component therapy has a central therapeutic role in clinical medicine, but blood banking and transfusion medicine has tended to focus on the blood component supply rather than the demand/patient perspective. The clinical focus should naturally be on ‘what is best for the patient?’ not, ‘what is best for the blood supply?’
Traditionally, transfusion has been regarded as the ‘default’ decision when there is clinical uncertainty. The benefits of transfusion have been assumed with little or no evidence to support the assumption and patients are thereby unnecessarily exposed to potential morbidity or even mortality. Given that the decision-making process for using blood component therapy can be difficult, that indications may be controversial or when there is no evidence for potential benefit, there are good common sense and scientifically evidence-based reasons to adopt a non-transfusion default position.1,2 If allogeneic blood component therapy can be avoided, the potential hazards cease to be an issue.
In considering the use of allogeneic blood transfusion the following questions need to be addressed:
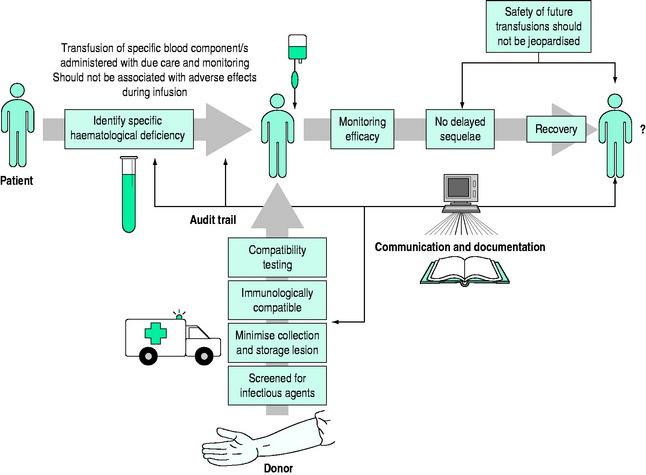
Safe and effective transfusion requires attention to the following details (Figure 88.1):
BLOOD STORAGE AND THE STORAGE LESIONS
Blood is altered from the moment of collection and the ‘lesions’ of collection – anticoagulation, separation, cooling, preservation and storage – compound and progressively increase until the date of expiry.3 The extent of these changes is determined by collection technique, the specific blood component, the preservative medium, the container, storage time and storage conditions. The threshold storage time for blood components has generally been arbitrarily determined by in vitro studies and assessment of in vivo survival. In the case of red cell concentrates, greater than 75% of transfused cells should survive post transfusion.
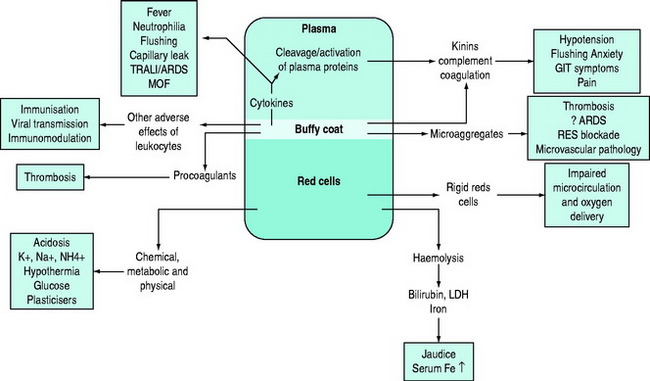
Storage results in quantitative and/or qualitative deficiencies in blood components, which may reduce the immediate efficacy of a transfusion. In parallel with these storage changes is an accumulation of degenerate material (e.g. microaggregates and procoagulant material), release of vasoactive agents, cytokine generation and haemolysis.4 Many of the changes occurring during storage are related to the presence of leukocytes and can be minimised by pre-storage leukoreduction.5 Red cells undergo a change from their biconcave disc shape to spiky spherocytes (echinocytes) and in so doing lose their flexibility. There are also changes in the red cell membrane resulting in an increased tendency to adhere to endothelial cell surfaces in the microcirculation, especially if there is activation of endothelial cells, for example in the presence of the systemic inflammatory response (e.g. with shock or sepsis).6 There is evidence that the immediate post-transfusion function of stored red cells and haemoglobin in delivering oxygen to the microcirculation and unloading is questionable, and several hours are required for red cell oxygen carriage and delivery to return to normal.7,8 It is important to differentiate between the storage lesion being responsible for failure to achieve clinical/laboratory end-points due to reduced survival and/or qualitative defects in cellular function and the ‘toxic’ effects of blood storage (Figure 88.2).
The use of blood filters has been an acknowledgement of the existence of the blood storage lesion and its possible clinical significance. The 170 μm blood-giving filters were first introduced into transfusion medicine to stop the occlusion of blood-giving sets. Ironically, there was little concern that the fibrin clots may harm the patient but fortunately the lung is one of nature’s remarkable filters. Adult respiratory distress syndrome (ARDS) and the Vietnam War increased interest in unfiltered microaggregates accumulating during storage. Both logic and animal data suggested their implication in ARDS and that microfilters to remove microparticles 20–40 μm in size may be protective. This proved difficult to confirm but microaggregate filters do not adequately address the problem of the storage lesion and its clinical significance. Using pre-storage leukodepletion filters, and preventing the development of the storage lesion in both blood and platelets from its inception, is more logical and scientific. Universal pre-storage leukoreduction is now standard practice in many countries, although it was primarily introduced as a precautionary measure against the possible transmission of variant Creutzfeldt–Jakob disease (vCJD) and not to address the numerous other indications for the removal of leukocytes.9
The clinical significance of blood storage lesions is still controversial. Further studies are needed to assess their relevance in conditions such as ARDS, multiorgan failure (MOF), vasoactive reactions and alterations in laboratory parameters.7,10,11 It is assumed that blood components have been appropriately collected, processed, stored, transported and transfused, but despite much greater attention to standard operating procedures and regulation generally, the quality of the final product cannot be guaranteed.12 The ‘assumed’ quality of labile cellular blood products is based on research data and monitoring of standard operating procedures. There is rarely detailed individual product assessment prior to transfusion.8 It is accepted that the adverse effects of storage increase with time and an arbitrary ‘cut off’ is mandated on the basis of research studies.
THE ROLE OF LEUKOCYTES AS A ‘CONTAMINANT’ IN LABILE STORED BLOOD AND THE ROLE OF PRE-STORAGE LEUKODEPLETION
There are several difficulties assessing potential adverse effects of leukocytes as they may be responsible for a wide range of blood component quality and safety issues.9 The presence of leukocytes has been shown to be responsible for specific adverse outcomes in some patients (e.g. non-haemolytic febrile transfusion reaction, platelet refractoriness and transfusion-associated graft-versus-host disease, TAGVHD), but this is the minority. In the larger context the overall available evidence, in the absence of adequate large randomised clinical trials, suggests that universal pre-storage leukodepletion may reduce transfusion-related morbidity and mortality as well as generating cost savings.13 Leukodepletion of red cell and platelet concentrates minimises the clinical consequences of the immunomodulatory effects of allogeneic transfusion. Hence it may decrease the incidence of recurrence of some cancers, of postoperative infections, of blood stream infections and reduce ICU and hospital length of stay. In many patients transfusion-related acute lung injury (TRALI) is a multifactorial disorder and in ‘at risk’ patients non-leukodepleted blood may be a risk factor. Patients in whom there is activation of the systemic inflammatory response syndrome (SIRS) are at risk of developing the multiorgan failure syndrome.5,14 Patients at particular risk include those with trauma, burns, critical bleeding, shock, sepsis and those undergoing cardiopulmonary bypass.15–19 The quality and function of pre-storage leukodepleted red cell concentrates is better maintained on storage, ensuring better post-transfusion efficacy and survival.6,20
CLINICAL GUIDELINES FOR BLOOD COMPONENT THERAPY
RED CELL TRANSFUSIONS
What constitutes appropriate use of red cell transfusions in acute medicine is contentious because of the difficulties in identifying the benefits of red cell transfusion in many circumstances.21 Pursuit of the lowest safe haematocrit continues to receive considerable attention, but pushing any aspect of a system to its limits risks ‘sailing too close to the wind’ which may be appropriate in some situations, but potentially hazardous in others.22,23
PLATELET TRANSFUSIONS
Platelet transfusion therapy may benefit patients with platelet deficiency or dysfunction. The following are the indications for platelet transfusions:24
PROPHYLAXIS
BLEEDING PATIENT
The transfusion of platelet concentrates is not generally considered appropriate:
FRESH FROZEN PLASMA
There are few specific indications for fresh frozen plasma, but its use may be appropriate:25
The use of fresh frozen plasma is generally not considered appropriate in cases of:
IMMUNOGLOBULIN
Normal human immunoglobulin is available in intramuscular and intravenous forms for the treatment or prevention of infection in patients with proven hypogammaglobulinaemia.29 Intravenous immunoglobulin G has been recommended as adjunctive therapy in patients with fulminant sepsis syndrome, especially those with toxic shock syndrome caused by group A streptococci.30
Intravenous immunoglobulin therapy also has a role in therapy of some autoimmune disorders, such as idiopathic thrombocytopenic purpura, autoimmune polyneuropathy and others (see Chapter 90).
FACTOR CONCENTRATES
There is an increasing number of plasma protein concentrates available for clinical use. Some are prepared from donor plasma and some by recombinant technology. Factor VIII and factor IX concentrates have an established role in the management of haemophilia, but others are in the process of establishing their clinical efficacy and indications. Antithrombin III (ATIII) concentrates are available for thrombophilia due to ATIII deficiency and are increasingly recommended in other disorders where ATIII may be depleted (e.g. DIC, MOF).31 Recombinant human activated protein C has antithrombotic, anti-inflammatory and profibrinolytic properties but its role in the treatment of patients with severe sepsis remains controversial.32
Of recent interest is the use of the recombinant activated haemostatic proteins and inhibitors. Recombinant activated factor VII (rFVIIa) was developed for the management of haemophilic patients with coagulation factor inhibitors. rFVIIa is now being widely used ‘off label’ for controlling haemorrhage in the non-haemophilic setting and there is considerable controversy as to its benefits and risks.33 There are an increasing number of case reports and series reporting on the use of rFVIIa in critical life-threatening haemorrhage. Patients with uncontrolled critical bleeding and coagulopathy, despite large transfusions and surgical intervention, have significant mortality rates and rFVIIa in these clinical situations is used as salvage therapy. rFVIIa is now being studied in controlled trials where there is critical bleeding in various clinical settings. rFVIIa initiates the extrinsic coagulation pathway when complexed to tissue factors at sites of injury and on activated platelet surfaces. It potentially has a role in a wide range of haemostatic disorders (e.g. massive blood transfusion, liver disease, uraemia, severe thrombocytopenia and platelet disorders).34
POTENTIAL ADVERSE EFFECTS OF ALLOGENEIC TRANSFUSION
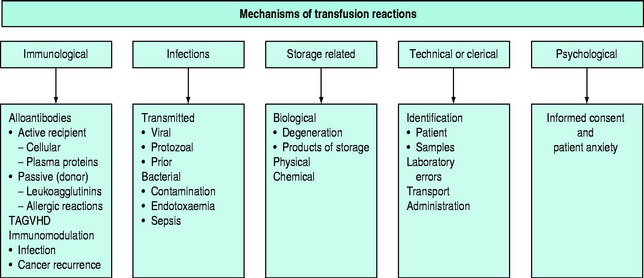
The pathophysiology of blood transfusion reactions can broadly be divided into five categories (Figure 88.3):
In terms of causation of an adverse clinical event, the possible role of blood transfusion can be classified into three categories on the basis of probability (Figure 88.4):

Full access? Get Clinical Tree