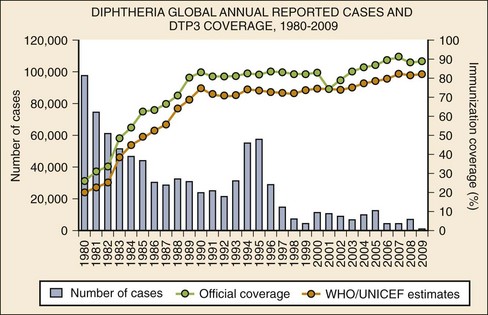
Chapter 129 In the fifth century BC, Hippocrates provided the first clinical description of what is likely diphtheria, characterized by sore throat, membrane formation, and death through suffocation, but references to the disease date to ancient Syria and Egypt. Epidemics of “throat distemper” occurred throughout the 16th, 18th, and 19th centuries. In 1821, French physician Pierre Bretonneau named the condition diphtherite, from the Greek word for leather, to describe the characteristic pharyngeal membrane. Klebs observed the Corynebacterium diphtheriae microorganism on smears obtained from pharyngeal membranes in 1883, and 1 year later Löffler isolated the organisms in pure cultures. Löffler subsequently demonstrated that diphtheria is a localized infection and postulated that an elaborated toxin caused its systemic effects. In 1888 Roux and Yersin demonstrated that bacteria-free filtrates of diphtheria culture were able to kill guinea pigs.1 In 1890, von Behring and Kitasato first demonstrated diphtheria immunization with a heat- and formalin-treated toxin to make toxoid. One year later they administered the first dose of antitoxin to a human with diphtheria. Schick developed the skin test for diphtheria immunity in 1913. During the 1930s and 1940s, toxoid immunization was routinely used. In the 1950s, Freeman found that only bacteria infected with the B phage produced toxin. Subsequent studies elucidated the toxin genome and the mechanism of toxin activity at the cellular level.1 Humans are the only known reservoir for C. diphtheriae. Spread is primarily by person-to-person contact through respiratory droplets or by direct contact with skin lesion exudates. Transmission is associated with crowded living conditions. Individuals may spread the disease when they are actively ill, in the convalescent stage after acute illness, or as asymptomatic carriers. Fomites and foods have occasionally been implicated but do not represent a major route of transmission.2 Between 1991 and 1996 the first large-scale epidemic of diphtheria in an industrialized country in three decades occurred in the newly independent states of the former Soviet Union, where the disease had previously been well controlled. During the peak of the epidemic, more than 98,000 cases and 3400 deaths were reported. Several factors contributed to this outbreak, including (1) decreased childhood immunity due to vaccine supply interruption and administration of adult-formulation tetanus-diphtheria toxoids (Td) to children, (2) increased adult susceptibility due to waning immunity, (3) poor socioeconomic conditions and increased population movement, and (4) resurgence of more toxigenic strains of diphtheria.3 Diphtheria remains endemic in many parts of the world and continues to cause sporadic cases and outbreaks.2 Immunization against diphtheria is highly effective (Fig. 129-1). Before widespread immunization programs in the United States, the incidence of diphtheria was in excess of 100 cases per 100,000 population, and the disease predominantly affected children. During this time, 80% of people acquired natural immunity to diphtheria by the age of 15 years, and recurrent exposure to toxigenic strains of the bacteria acted as a booster. Because childhood immunization nearly eliminates these toxigenic strains in a population, adult immunity wanes. Thus more adults in industrialized nations are susceptible to diphtheria.4 By the 1980s the Centers for Disease Control and Prevention (CDC) reported only 0 to 5 cases per year nationwide. Currently, sporadic cases occur primarily in adults, many of whom are not adequately immunized.3 Three urban outbreaks of predominantly cutaneous diphtheria occurred in Seattle between 1972 and 1982 among a population of urban alcohol abusers. Outbreaks are associated with poor hygiene, crowding, underlying skin disease, contaminated fomites, pyoderma, and the appearance of new C. diphtheriae strains.1 Even in industrialized nations in which childhood vaccination rates are high, more than 50% of adults older than 40 years lack protective antibodies.4 The ease of international travel and the epidemic in eastern Europe in the 1990s underscore the importance of aggressive continuation of childhood immunizations and reimmunization of adults. Diphtheria is caused by C. diphtheriae, an unencapsulated, nonmotile, gram-positive bacillus named for its shape (korynee, for “club”) and for its characteristic clinical presentation (diphtheria, for “leather hide,” referring to the appearance of the leathery pharyngeal membrane). When they are viewed on stained smears, the bacteria look like Chinese characters.1 Infection by C. diphtheriae can occur at various sites of the respiratory tract or the skin. Respiratory diphtheria includes faucial (pharyngeal or tonsillar), nasal, and laryngeal (tracheobronchial) types, named for the primary location of infection. Cutaneous diphtheria can occur as a primary skin infection or as a secondary infection of a preexisting wound.1 The C. diphtheriae bacterium produces an exotoxin that contributes to formation of the diphtheritic membrane and is responsible for the systemic effects of infection. The exotoxin is a 62,000-dalton polypeptide produced by bacterial strains lysogenized by the corynephage B tox+.1,5 The exotoxin inhibits cellular protein synthesis. Circulating exotoxin most profoundly affects the nervous system, heart, and kidneys.1 The degree of local and systemic toxicity depends on the location and extent of membrane formation. Pharyngeal diphtheria generally has the greatest toxicity and cutaneous diphtheria the least. The diphtheritic membrane forms as a result of necrosis caused by the local effects of the exotoxin. The membrane is composed of leukocytes, erythrocytes, fibrin, epithelial cells, and bacteria. Initially, the pharynx appears erythematous, but as necrosis occurs, grayish white patches appear and eventually coalesce. The membrane is accompanied by surrounding edema and cervical adenitis. The initial grayish white, filmy appearance changes to a thick, grayish black membrane with sharply defined borders. This membrane adheres to the underlying tissue, and bleeding occurs if removal is attempted.1 Systemic effects of diphtheria infection are caused by the circulating exotoxin’s action primarily on the cardiovascular and nervous systems. The exotoxin disrupts cellular protein synthesis and produces a peripheral neuropathy manifested by muscle weakness. About 5% of all patients with symptomatic respiratory infection will have polyneuritis, but 75% of patients with severe disease have some form of neuropathy.6 The muscles of the palate are usually the first to become paralyzed. Less commonly, other cranial nerves, peripheral nerves, and the spinal cord are affected. Degenerative lesions develop in the dorsal root and ventral horn ganglia of the spinal cord and in cranial nerve nuclei. Cortical cells are spared. Proximal muscle groups are affected first. In severe cases, paralysis may develop in the first few days of illness. In general, the paralysis does not last more than 10 days, and complete recovery during a longer time is the rule.1 The extent of cardiac complications correlates with the degree of local infection and membrane formation. Signs of myocardial dysfunction usually appear 1 to 2 weeks after the onset of illness. In more severe cases, cardiac symptoms arise earlier in the course of the illness. The exotoxin directly damages myocardial cells, producing myocarditis. Electrocardiographic changes suggestive of myocarditis occur in up to two thirds of patients, but clinical manifestations of myocarditis are less common (10-25%).1 The average incubation period of respiratory tract diphtheria is 2 to 4 days but may range from 1 to 8 days. Signs and symptoms are often indistinguishable from those of other upper respiratory tract infections. In a series of 676 patients, fever and sore throat were the most frequent presenting complaints (79% and 69%, respectively). Weakness (42%), dysphagia (35%), headache (20%), change of voice (15%), and loss of appetite (10%) were also common. Cough, shortness of breath, nasal discharge, and neck edema occurred in less than 10% of patients. Fever, although common, is usually low grade. Cervical adenopathy is present in approximately one third of patients, and a diphtheritic membrane is observed in more than half of all patients. Of note, however, one report indicated that shortness of breath and neck edema were present in approximately 40% of patients who died of the disease.6 In patients with faucial diphtheria, the extent of the membrane usually parallels the clinical toxicity. If the membrane is limited to the tonsils, the disease may be mild; if the membrane covers the entire pharynx, the onset of illness is usually abrupt and the disease severe. Swelling of the cervical lymph nodes and infiltration of tissues of the neck may be so extensive that the patient has a “bull-neck” appearance. Patients with this form of “malignant diphtheria” usually have high fever, severe muscle weakness, vomiting, diarrhea, restlessness, and delirium.1,6,7 Death occurs from respiratory tract obstruction or cardiac failure from myocarditis. Nasal diphtheria arises with a unilateral or bilateral serous or serosanguineous discharge from the nose. A diphtheritic membrane may be visible. These patients do not usually have constitutional symptoms. Treatment is important to prevent a persistent carrier state. Laryngeal (tracheobronchial) diphtheria may begin in the larynx or spread downward from a more cephalad primary site. Respiratory tract edema with subsequent upper airway obstruction may develop. Patients with cutaneous diphtheria generally do not display systemic toxicity. The skin characteristically has an ulcer with a grayish membrane. Wounds from which C. diphtheriae is cultured are clinically indistinguishable from other chronic skin conditions.1 The most serious complications of diphtheria are airway obstruction (resulting from membrane formation and edema), congestive heart failure, cardiac conduction disturbances, and muscle paralysis. Mortality in two large series ranged from 2 to 3% overall but was up to 7% in patients with myocarditis and 26% in patients with the malignant form of the disease (with neck swelling).7 Unimmunized and underimmunized children requiring intensive care have higher mortality rates (78%) from myocarditis and often develop renal failure.8 Although systemic infection is rare, endocarditis, mycotic aneurysms, osteomyelitis, and septic arthritis have all been described in immunocompromised hosts.1 When C. diphtheriae is suggested, the laboratory should be notified because routine cultures do not identify the organism. Throat or nasopharyngeal swabs should be obtained for respiratory diphtheria, and if it is present, membranous material should be examined. For cutaneous infections, samples should be obtained from skin lesions. Specimens should be collected before antibiotic therapy is initiated and should be transported to the laboratory immediately for rapid inoculation onto tellurite (Tinsdale’s) or Löffler’s selective culture medium.1 Immunofluorescent staining of a 4-hour culture may provide a rapid diagnosis, but direct staining is frequently unreliable. Definitive identification is made by use of a combination of colony morphology, microscopic appearance, and fermentation reactions.1 C. diphtheriae isolates should be tested for the production of toxin. The Elek immunoprecipitation test for toxin A is technically demanding and subject to misinterpretation by inexperienced users. Polymerase chain reaction (PCR), which is more reliable but not as readily available, can be used to detect the diphtheria toxin structural gene. Newer methods that rapidly detect the toxin by mass spectrometry are not readily available but may be used in the future.9 A positive culture for group A beta-hemolytic streptococcus does not exclude diphtheria as a pathogen because up to 30% of patients with diphtheria test positive for streptococcal coinfection or carrier state. Several laboratory abnormalities, such as leukocytosis, mild thrombocytopenia, and proteinuria, are common but are neither sensitive nor specific for diphtheria. Electrocardiographic changes are nonspecific and include ST-T wave changes, varying degrees of atrioventricular block, and dysrhythmias. An electrocardiogram may be normal even in the presence of myocarditis. An echocardiogram may show dilated or hypertrophic cardiomyopathy. Cardiac enzymes may be elevated, and serum troponin levels correlate with the severity of myocarditis.10 In the absence of a diphtheritic membrane, it may be difficult to differentiate respiratory diphtheria from many other respiratory conditions, especially in the early phase of infection (Box 129-1). In general, the diphtheritic membrane is darker, grayer, more fibrous, and more firmly attached to the underlying tissues than in other conditions that have a membrane-like appearance. Vincent’s angina frequently involves the gingivae, which are unaffected in diphtheria. Acute bacterial epiglottitis generally has a much more rapid onset than diphtheria, and indirect laryngoscopy reveals an erythematous, edematous epiglottis without membrane formation.1 Patients with clinical evidence of diphtheria should be placed in respiratory isolation and treated presumptively for C. diphtheriae infection. The goals of therapy are to protect the airway, to limit the effects of already produced toxin, and to eliminate future toxin production by terminating the growth of C. diphtheriae. Although the likelihood for the development of airway obstruction from diphtheria is remote for a patient in the United States, the management is identical to that for other forms of airway obstruction. Bronchodilators may be useful in symptomatic patients.10 Patients may be dehydrated from fever and decreased oral intake related to dysphagia or neurologic impairment. Fluid resuscitation should be undertaken cautiously as the toxin’s effect on the myocardium may result in congestive heart failure.1,10 Equine serum diphtheria antitoxin (DAT) should be administered promptly after the clinical diagnosis of respiratory diphtheria is made and before laboratory confirmation.10–12 DAT is not licensed by the Food and Drug Administration (FDA) for use in the United States. The CDC is authorized to distribute DAT to physicians as an investigational new drug. DAT can be obtained by contacting the CDC Emergency Operations Center at 770-488-7100. The diphtheria duty officer can also be contacted at 404-639-3158 during duty hours.12 The size and location of the membrane, the duration of illness, and the patient’s overall degree of toxicity determine the dosage of antitoxin. The CDC recommends 20,000 to 40,000 units for pharyngeal or laryngeal involvement of 48 hours’ duration, 40,000 to 60,000 units for nasopharyngeal lesions, and 80,000 to 120,000 units for systemic disease of 3 days’ duration or more or for diffuse swelling of the neck.12 After conjunctival or intradermal sensitivity skin testing, the antitoxin is administered intravenously. If the patient exhibits sensitivity to the antitoxin, desensitization should be performed. Active immunization against diphtheria should also be initiated because clinical infection does not necessarily confer immunity.10 Antibiotics are beneficial in preventing growth and spread of the organism but are no substitute for antitoxin. Erythromycin 30 to 50 mg/kg/day (up to 2 g) intravenously (IV) or orally in divided doses, aqueous crystalline penicillin 100,000 to 150,000 units/kg/day in four divided doses intramuscularly (IM), and procaine penicillin 25,000 to 50,000 units/kg/day in two divided doses for 14 days given IM are acceptable alternatives.10 Treatment failures are slightly more common with penicillin than with erythromycin. The newer generation macrolides (azithromycin and clarithromycin) have activity similar to that of erythromycin in vitro and may result in better compliance. These agents have not yet been adequately tested in clinical disease. An equivalent daily oral therapy may be substituted when the patient is able to swallow. Negative cultures should be documented after treatment.10 Cutaneous lesions should be débrided of necrotic tissue and cleansed vigorously. A course of antibiotics is recommended, but the administration of antitoxin for cutaneous lesions is of questionable value. Some experts recommend 20,000 to 40,000 units of antitoxin, but few data support its use in this setting.1,10 Carriers of C. diphtheriae should receive oral penicillin G or erythromycin for 7 days or intramuscular benzathine penicillin (600,000 units for those weighing less than 30 kg and 1,200,000 units for those weighing more than 30 kg). Active immunization should also be provided to unimmunized and partially immunized carriers. After 2 weeks of therapy is completed, cultures should be obtained; if cultures are positive, erythromycin therapy should be given for 10 additional days.1 Individuals who have been in close contact with infected patients should have culture specimens taken, and the patient should be kept under surveillance for 7 days. Previously immunized close contacts should receive a booster of diphtheria toxoid if the last booster was more than 5 years earlier. The vaccine should be diphtheria, tetanus, and acellular pertussis (DTaP), diphtheria-tetanus (DT), or tetanus-diphtheria with a lower dose of diphtheria toxoid (Td) as appropriate for age according to the recommended immunization schedule. Close contacts who are not immunized or whose immunization status is unknown should receive the same antimicrobial therapy as carriers (as previously described), have culture specimens taken before and after therapy, and have active immunization initiated. Close contacts who cannot be kept under surveillance should receive benzathine penicillin intramuscularly to ensure compliance and a Td booster (appropriate for age and immunization history). Some practitioners treat this group with 5000 to 10,000 units of antitoxin intramuscularly (at a site separate from the toxoid booster) after sensitivity testing. This is generally not recommended, however, because of the risk of horse serum allergy.12 Pertussis is an acute respiratory disease that was first described in 1578 when an epidemic swept through Paris. The name pertussis was first used by Sydenham in 1670 when he described the illness in infants. Pertussis literally means “violent cough,” which is the hallmark of the disease. In China it is known as “the cough of 100 days.” It is also called whooping cough because the severe episodes of coughing are followed by forceful inspiration, which creates the characteristic whooping sound. The causative organism was identified in 1906 by Bordet and Gengou.13 In the prevaccination era, pertussis was a major cause of mortality among infants and children in the United States. A vaccine was developed in the 1940s, but pertussis still remains a significant cause of morbidity and mortality in the United States and worldwide. Pertussis is a localized respiratory illness transmitted by aerosolized droplets. It is highly contagious, with attack rates greater than 50% in adults exposed more than 12 years after completion of a vaccination series and up to 90% in susceptible individuals with a household exposure.14 The average incubation period is 7 to 10 days but may range from less than 1 week to 3 weeks. Neither vaccination nor prior infection confers lifelong immunity. Pertussis remains prevalent worldwide, with 106,207 cases reported to the World Health Organization in 2009 (Fig. 129-2A). In the United States, annual pertussis rates declined sharply after the introduction of the vaccine and reached a nadir of 1010 cases in 1976. Since then, there has been a steady increase in the incidence of pertussis, with 11,647 cases reported in 2003 and 25,616 in 2005 (Fig. 129-2B).14 Waning immunity in the adult population and increased reporting of adult cases may be contributing factors. A 1991 report found evidence of a causal relationship between the vaccine and acute encephalopathy. Although there appears to be no causal relationship between the vaccine and long-term neurologic complications, the report resulted in a decline in the use of the whole-cell pertussis vaccine. The acellular pertussis vaccine has been approved in the United States since 1991 for persons 15 months to 64 years and since 1997 for infants.14 Figure 129-2 A, Global annual reported cases of pertussis compared with percentage of immunization coverage. DTP3, third dose of diphtheria-tetanus-pertussis vaccine. Pertussis is caused by organisms of the Bordetella genus, which are small, aerobic, gram-negative coccobacilli that occur singly or in pairs. Bordetella pertussis and Bordetella parapertussis are primarily responsible for disease in humans. The organisms are fastidious and require nicotinamide and an optimal temperature of 35 to 37° C to grow. Bordetella bronchiseptica, a flagellated, motile organism, causes illness in animals, including kennel cough, and may rarely cause respiratory infection in immunocompromised humans.13 The Bordetella organism adheres preferentially to ciliated respiratory epithelial cells. B. pertussis does not invade beyond the submucosal layer in the respiratory tract and is almost never recovered in the bloodstream. The organism elaborates several toxins that act locally and systemically. These toxins include pertussis toxin, dermonecrotic toxin, adenylate cyclase toxin, and tracheal cytotoxin.13 Local tissue damage consists of inflammatory changes in the mucosal lining of the respiratory tract, primarily congestion and cellular infiltration with lymphocytes and granulocytes. As the infection progresses, secondary pneumonia or otitis media may occur. Systemic effects of pertussis toxin include sensitization to the lethal effects of histamine and increased secretion of insulin. This hyperinsulinemia can cause hypoglycemia, particularly in young infants.13 Pertussis arises in three distinct sequential clinical stages: the catarrhal phase, the paroxysmal phase, and the convalescent phase. The catarrhal or prodromal phase begins after an incubation period of approximately 7 to 10 weeks and lasts approximately 1 to 2 weeks. Infectivity is greatest during the catarrhal phase, when the disease is clinically indistinguishable from other upper respiratory tract infections. Signs and symptoms include rhinorrhea, low-grade fever, malaise, and conjunctival injection, which are clinically indistinguishable from a common upper respiratory tract infection. A dry cough usually begins at the end of the catarrhal phase.13 The paroxysmal phase begins as fever subsides and cough increases and lasts 2 to 4 weeks. Paroxysms of staccato coughing occur 40 to 50 times per day. The patient coughs repeatedly in short exhalations, followed by a single, sudden, forceful inhalation that produces the characteristic “whoop.” Only one third of adults with pertussis develop this whoop, and it is rare in young infants, who may present with apneic episodes and no other symptoms. Paroxysms may be spontaneous, occur more frequently at night, or be precipitated by noise or cold. During the paroxysm, the patient may exhibit cyanosis, diaphoresis, protrusion of the tongue, salivation, and lacrimation. Post-tussive vomiting, syncope, and brief episodes of apnea may occur. Infants may be physically exhausted after a typical paroxysm. Between episodes of coughing, patients do not appear acutely ill.13 Atypical presentations may occur in young and preterm infants. Fever is usually not present in uncomplicated neonatal pertussis. Tachypnea, apnea, and cyanotic and bradycardic episodes may be the predominant symptoms.15 Older children and adults who have partial protection from vaccination or previous illness may have a long-lasting intractable dry cough that is frequently misdiagnosed as bronchitis. Post-tussive vomiting in adults is highly suggestive of pertussis.13 Physical examination findings are nonspecific. Tachypnea is variably present and may be related to the degree of pulmonary involvement. Low-grade fever is common during the catarrhal phase, as are conjunctival injection and rhinorrhea. The presence of fever during other stages of illness suggests secondary infection. Petechiae above the nipple line, subconjunctival hemorrhages, pneumothorax, and epistaxis may occur because of increased intrathoracic pressure during coughing paroxysms.13,15 Chest examination may reveal rhonchi or clear lung fields; the presence of rales suggests pneumonia. The major complications of pertussis are pneumonia superinfection, central nervous system (CNS) sequelae, otitis media, and complications related to the paroxysm of coughing. Pneumonia complicating pertussis is a leading cause of death, especially in infants and young children.13–18 Aspiration of gastric contents and respiratory secretions may occur during the paroxysm of coughing, whooping, and vomiting. Secondary pulmonary infection may also be a consequence of decreased respiratory tract clearance related to the actions of the Bordetella organism and its toxins on bronchial and lung mucosa. Bacterial (Streptococcus pneumoniae, Streptococcus pyogenes, Haemophilus influenzae, and Staphylococcus aureus) and viral (respiratory syncytial virus, cytomegalovirus, and adenovirus) superinfections can complicate pertussis infections. A fever during the paroxysmal phase should alert the physician to a possible superinfection. CNS complications include seizures and encephalopathy in about 1%.19 The causes are unclear but may include hypoxia, hypoglycemia, cerebral petechia, effects of a toxin, or secondary infection by neurotropic viruses or bacteria. CNS hemorrhages may occur as a consequence of the increased cerebrovascular pressures generated during the paroxysm of coughing. Sudden increases in intrathoracic and intra-abdominal pressures can result in several other complications (Box 129-2).13–19 Bradycardia, hypotension, and cardiac arrest can occur in neonates and young infants with pertussis. Severe pulmonary hypertension has increasingly been recognized in this age group and can lead to systemic hypotension, worsening hypoxia, and increased mortality.15,16 Intensive care monitoring is recommended for these patients, regardless of how well they may appear on admission. The diagnosis of pertussis should be entertained in any patient with prolonged cough with paroxysms, whoops, or post-tussive emesis, regardless of previous vaccination status. Up to 25% of adults in the United States who have a prolonged cough have serologic evidence of pertussis.13 Ancillary studies are of limited value in the emergency department. During the late catarrhal and early paroxysmal phases, a marked leukocytosis and a characteristic lymphocytosis are often present. The white blood cell (WBC) count of 25,000 to 50,000/mL is not uncommon and may exceed 100,000/mL in infants.17 Adults with pertussis frequently do not have the characteristic leukocytosis and lymphocytosis, and some infants and immunocompromised hosts may not be able to mount this response. The chest radiograph may show peribronchial thickening, atelectasis, or pulmonary consolidation.18 Laboratory confirmation of the diagnosis is made by nasopharyngeal culture and PCR, if both are available; sputum and throat swabs are inadequate.13 The Bordetella organism is fastidious, and isolation requires a nicotinamide or Bordet-Gengou medium impregnated with antibiotics to reduce overgrowth of competing bacteria. The slow-growing hemolytic colonies of B. pertussis take 3 to 7 days to appear. A synthetic culture medium is also available. The sensitivity of pertussis cultures is only 15 to 80% and drops to only 1 to 3% three weeks after the onset of cough. Direct fluorescent antibody techniques are no longer recommended to identify B. pertussis. Adults generally come to medical attention late in the disease, at which time cultures are rarely positive (3.6%). PCR is much more likely to identify the organism but has a high false-positive rate. Most laboratories use enzyme-linked immunosorbent assay, which rises 2 to 3 weeks after infection or primary immunization. Paired serologic tests showing a twofold increase are the “gold standard” for diagnosis.13 Treatment of pertussis is primarily supportive and includes oxygen, frequent suctioning, appropriate hydration, parenteral nutrition if necessary, and avoidance of respiratory irritants. Patients with suggested pertussis and associated pneumonia, hypoxia, or CNS complications or those experiencing severe paroxysms should also be hospitalized. Children younger than 1 year should also be admitted because they are not yet fully immunized and have the greatest risk for morbidity and mortality. Neonates with pertussis should be admitted to an intensive care unit (ICU) as apnea and significant cardiac complications can occur without warning.15–17 Antibiotic treatment does not appear to significantly reduce the severity or duration of illness when it is started in the paroxysmal phase and may have only a minimal effect in the catarrhal phase. The primary goal of antibiotic therapy is to decrease infectivity and carriage. Erythromycin estolate ester is the antibiotic of choice at 40 to 50 mg/kg/day (maximum 2 g/day) in two or three divided doses for 14 days.13,18–20 Azithromycin (10 mg/kg on day 1, followed by 5 mg/kg on days 2-5), clarithromycin (15 mg/kg/day in two divided doses), and a 7-day course of erythromycin estolate ester are effective alternatives for patients who do not tolerate 14 days of erythromycin.13,18,19 Trimethoprim-sulfamethoxazole (8 mg/kg/day of trimethoprim) is an alternative for macrolide-allergic patients, but efficacy is unproven. Patients should be considered infectious for 3 weeks after the onset of the paroxysmal phase or until at least 5 days after antibiotics are started.13 Strict droplet isolation is recommended during this period. Corticosteroids, especially in young critically ill infants, may reduce the severity and course of illness, but effectiveness is not well established. Beta2-adrenergic agonists do not reduce the frequency or severity of paroxysmal coughing episodes but may be helpful in patients with reactive airway disease. Trials with pertussis immune globulin are limited and to date show no proven benefit.21 Standard cough suppressants and antihistamines are ineffective. Postexposure prophylaxis with erythromycin as described previously should be considered for infants younger than 6 months who are household contacts of infected patients.13 Erythromycin may also be prescribed for any unimmunized person or partially immunized infant with a history of significant exposure to the index case. There is no clinical benefit of prophylaxis for exposed patients older than 6 months, and medication side effects are common.13,20 Whole-cell and acellular pertussis vaccines are distributed in combination with diphtheria and tetanus toxoids as DPT and DTaP, respectively. The acellular pertussis vaccines contain inactivated pertussis toxin and one or more other bacterial components. The acellular vaccines are slightly less effective than the whole-cell vaccine but have fewer reported adverse reactions.22,23 Most recipients have fever, irritability, behavioral changes, and local discomfort at the site of inoculation. Moderately severe reactions are uncommon but include fever with temperature above 40° C, persistent crying, high-pitched crying, and seizures. Severe neurologic complications (prolonged seizures and encephalopathy) occur rarely but led to decreased use of the whole-cell form of the vaccine and the development of DTaP.23 DTaP has replaced DPT for childhood immunizations, and the whole-cell pertussis vaccine is recommended for use in the United States only when the acellular vaccine is not available.23 Tdap (with reduced diphtheria toxoid and acellular pertussis) is indicated as a booster vaccine in persons aged 10 to 64 years. There are no Tdap vaccines currently licensed for use in persons older than 65 years. The CDC Advisory Committee on Immunization Practices (ACIP) does, however, recommend that persons older than 65 years who have never received Tdap and anticipate close contact with infants younger than 12 months receive a single dose of Tdap, regardless of interval since last Td vaccination.24 Pertussis immunity wanes significantly 3 to 8 years after immunization and 15 years after natural infection, causing an increasing incidence of the disease in people older than 15 years. The acellular pertussis vaccine is safe and effective in adolescents and adults, and routine booster immunization is currently recommended by the CDC ACIP for persons aged 11 to 18 years.14 Not yet available, a nasal pertussis vaccine may more closely simulate natural infection with the potential of conferring longer immunity.22 Tetanus is a toxin-mediated disease characterized by severe uncontrolled skeletal muscle spasms. Involvement of the muscles of respiration leads to hypoventilation, hypoxia, and death. Dramatic descriptions of this disease date to ancient Egypt, when physicians recognized a frequent relationship between tissue injury and subsequent fatal spasm.25 In 1884, Carle and Rattone produced tetanus in rabbits by injecting material from an acne pustule that came from an infected human. In the same year, Nicolaier isolated the strychnine-like toxin from anaerobic soil bacteria. In 1889, Kitasato obtained pure cultures of spore-forming bacteria that caused tetanus on introduction into animals. One year later, Faber proved that tetanus is a toxin-mediated disease when he induced the illness by injecting animals with bacteria-free filtrates of Clostridium tetani cultures. In the 1890s, von Behring and Kitasato discovered tetanus antitoxin in the serum of immune animals and demonstrated its efficacy in preventing disease. Prophylactic injection of this antitoxin provided passive immunity to wounded soldiers during World War I. It was not until 1924 that an effective vaccine was developed by Descombey. Large-scale testing during World War II indicated that the tetanus toxoid confers a high degree of protection against disease.25–27 Despite the availability of an effective vaccine, tetanus remains endemic worldwide. It is more common in warm, damp climates and relatively rare in cold regions. The global annual incidence of reported cases of tetanus has declined steadily with the introduction of vaccination programs (Fig. 129-3A). The World Health Organization reported 9836 cases of tetanus in 2009, but it is estimated that 800,000 to 1 million unreported cases occur a year, with half occurring in neonates. Eighty percent of these cases occur in Africa and Southeast Asia because of low immunization rates and poor hygiene.27 Figure 129-3 A, Global annual reported cases of tetanus compared with percentage of immunization coverage. DTP3, third dose of diphtheria-tetanus-pertussis vaccine. Since the introduction of vaccination programs in the United States, the incidence of tetanus has steadily declined from 4 cases per million population in the 1940s to 0.095 case per million population in 2005 (Fig. 129-3B).28 The highest incidence occurs in people older than 65 years (0.23 case per million population), and the incidence in Hispanic Americans is almost twice that in non-Hispanics.28,29 Fifteen percent of cases occur in injection drug users. The overall case fatality rate is 18% but approaches 50% in patients older than 70 years (Fig. 129-4). Cases have been reported in patients who had been fully vaccinated, but in the eight patients from 1998 to 2000, no deaths occurred.29 Tetanus typically occurs as a result of a deep penetrating wound. A history of injury is present in more than 70% of patients, but the injury may be trivial in 50% of patients and unapparent in up to 30% of patients.25–29 The most common portals of entry for the organism are puncture wounds, lacerations, and abrasions. Tetanus has also been reported in association with chronic skin ulcers, abscesses, and otitis media as well as with foreign bodies, corneal abrasions, childbirth, and dental procedures.26 Postoperative tetanus has been reported in patients who have undergone intestinal operations and abortions. In these cases the source of bacteria is probably endogenous as up to 10% of humans harbor C. tetani in the colon. C. tetani is a spore-forming, motile, slender, rod-shaped, obligate anaerobic bacillus. It stains gram positive in fresh culture but has a variable staining pattern in old cultures and tissue samples. The bacillus can form a single spherical terminal endospore that swells the end of the organism to produce a characteristic drumstick appearance. C. tetani is ubiquitous in soil and dust and is also found in the feces of animals and humans.25 Mature bacilli are highly susceptible to heat and other adverse environmental conditions. Spores are resistant to heating and chemical disinfectants and can survive in soil for months to years. When they are introduced into a wound, spores may not germinate for weeks because of unfavorable tissue conditions. When injury favors anaerobic growth, the spores germinate into mature bacilli. Only these mature bacilli produce the tetanus toxin that causes clinical disease.25–27 C. tetani produces the neurotoxin tetanospasmin (TS) at the site of tissue injury. TS first binds the motor nerve ending and then moves by retrograde axonal transport and trans-synaptic spread to the CNS.30,31 It binds preferentially to inhibitory (GABAergic and glycinergic) neurons and blocks the presynaptic release of these neurotransmitters. Interneurons afferent to alpha motor neurons are affected first.30 Without inhibitory control, the motor neurons undergo sustained excitatory discharge, resulting in the muscle spasm characteristic of tetanus.26 TS may also affect preganglionic sympathetic neurons and parasympathetic centers, resulting in autonomic nervous system dysfunction.31,32 The clinical manifestations include dysrhythmias and wide fluctuations in blood pressure and heart rate. The binding of TS at the synapse is irreversible; recovery occurs only when a new axonal terminal is produced.25 The incubation period for tetanus ranges from 1 day to several months. A shorter incubation period portends a worse prognosis.26 The duration of the incubation period is not useful in making the diagnosis of tetanus because many patients have no history of an antecedent wound. Four types of clinical tetanus have been described. Generalized Tetanus.: Generalized tetanus is the most common form of the disease and results in spasms of agonist and antagonist muscle groups throughout the body. The classic initial presenting symptom of trismus (“lockjaw”) is caused by masseter muscle spasm and is present in 50 to 75% of patients. As the other facial muscles become involved, a characteristic sardonic smile (risus sardonicus) appears. Other early symptoms include irritability, weakness, myalgias, muscle cramps, dysphagia, hydrophobia, and drooling. As the disease progresses, generalized uncontrollable muscle spasms can occur spontaneously or as a result of minor stimuli, such as touch or noise. Spasms may result in vertebral and long bone fractures and tendon rupture. Opisthotonos is a prolonged tonic contraction that closely resembles decorticate posturing. Spasms of laryngeal and respiratory muscles can lead to ventilatory failure and death. Autonomic dysfunction is the major cause of death in patients who survive the acute phase and is manifested by tachycardia, hypertension, temperature elevation, cardiac dysrhythmias, and diaphoresis. The illness is progressive, with an increase in symptoms during the first 3 days, persistence of symptoms for 5 to 7 days, and reduction of spasms after 10 days. If the patient survives, recovery is complete after 4 weeks or more. Throughout the course of this horrific illness, patients remain completely lucid unless they are chemically sedated.25–27 Localized Tetanus.: Localized tetanus is a form of the disease characterized by persistent muscle spasms close to the site of injury. Symptoms may be mild or severe, but mortality is lower than with generalized tetanus. Local tetanus may progress to generalized disease. This form of illness may probably reflect partial immunity to TS and may be present for weeks to months before resolution.25 Cephalic Tetanus.: Cephalic tetanus is a rare variant of localized tetanus that results in cranial nerve palsies and muscle spasms. The palsies precede the spasm in 42% of cases, resulting in frequent misdiagnosis. The most commonly involved cranial nerve is the facial nerve (VII), mimicking Bell’s palsy. Most of these cases occur after facial trauma or otitis media. Patients have trismus and palsies of cranial nerve III, IV, VII, IX, X, or XII ipsilateral to the site of local infection. The clinical course is variable. In one third of cases, resolution of symptoms is complete. The remainder progress to generalized tetanus with an overall mortality rate of 15 to 30%.25–29 Neonatal Tetanus.: Neonatal tetanus is generalized tetanus of the newborn and occurs almost exclusively in developing countries where maternal immunization is inadequate and contaminated material is used to cut and dress umbilical cords. Symptoms begin during the first week of life and include irritability and poor feeding. Mortality approaches 100% because of the high toxin load for body weight and inadequate medical support in developing countries. Even with limited resources, mortality can be reduced to less than 50% with basic medication and experienced medical and nursing personnel.33 The CDC reported one case of neonatal tetanus in the United States between 1998 and 2000. The infant was born at home to an unimmunized mother. The umbilical cord had been treated with bentonite clay. The child was treated and recovered after 19 days of hospitalization. Acute respiratory failure, the main cause of morbidity and mortality in tetanus, results from respiratory muscle spasms or laryngospasms and airway obstruction. If the patient survives the acute onset of illness and has adequate ventilatory support, autonomic dysfunction becomes the leading cause of death. Autonomic instability occurs several days after the onset of generalized spasms. Disinhibition of the sympathetic nervous system predominates and causes dysrhythmias, hypertension, myocarditis, and pulmonary edema.34 Dysrhythmias and myocardial infarction are the most common fatal events during this phase. Secondary infection may occur in the initial inoculating wound or as a complication arising from invasive treatment modalities, such as mechanical ventilation.35 Hyperthermia may also result from muscle spasms and sympathetic hyperactivity. Prolonged immobility can lead to deep venous thrombosis and pulmonary embolism. Gastrointestinal complications include peptic ulcers, ileus, intestinal perforation, and constipation. The syndrome of inappropriate secretion of antidiuretic hormone occurs in a small number of patients. Hemolysis has also been reported. Mortality is a function of the previous immunization status, incubation period, severity and rapidity of onset of symptoms, comorbid disease, age, and sophistication of medical treatment available. With appropriate intensive care treatment, elder patients may fare as well as their middle-aged counterparts.36 Long-term physical complications in survivors are rare. The most common persistent problem may be psychological trauma related to the disease and its treatment.25 The diagnosis of tetanus should be made on clinical grounds alone. Wound cultures for C. tetani are of little value as they are positive in only one third of cases. Even if a positive culture is obtained, it does not indicate whether the bacterium is a toxin-producing strain. There are no laboratory tests to confirm or to exclude the diagnosis of tetanus.25 In 1990 the CDC adopted a clinical case definition for the public health surveillance of generalized tetanus: “acute onset of hypertonia or painful muscular contractions (usually of the muscles of the jaw and neck) and generalized muscle spasms without other apparent medical cause (as reported by a health care professional).29 Lumbar puncture may be indicated to exclude meningitis in the neonate when the diagnosis of tetanus is uncertain. A computed tomography scan is helpful in assessment for intracranial disease. A serum calcium level is helpful to exclude hypocalcemia. Electromyography may be useful if the diagnosis of cephalic or localized tetanus is in doubt.25 The spatula test involves touching of the oropharynx with a tongue blade. With a negative test result, the patient gags and expels the tongue blade. With a positive test result, the patient has reflex masseter muscle spasm and bites the spatula. This test is 94% sensitive and 100% specific for tetanus.27 Strychnine poisoning is the only clinical condition that truly mimics generalized tetanus. Strychnine, like TS, antagonizes glycine release, but unlike TS, it has no effect on γ-aminobutyric acid (GABA) release. Patients have opisthotonos while remaining alert. The annual incidences of tetanus and strychnine poisoning are similar in the United States, and serum and urine tests for strychnine should be performed when tetanus is considered.25 In patients who present with diffuse generalized spasm, the diagnosis of tetanus is less likely to be missed, but ideally the disease should be considered and diagnosed in the early stages to minimize complications and to decrease mortality. Some conditions with clinical similarities to tetanus are listed in Box 129-3. Trismus is most commonly caused by intraoral infections. These can be excluded with careful history and physical examination of the oral cavity and teeth. Mandibular dislocation can be ruled out with appropriate radiographs of the mandible and temporomandibular joints. Dystonic reactions can be differentiated from tetanus by medication history and symptoms that are alleviated by benztropine or diphenhydramine. Patients with encephalitis usually exhibit an altered mental status. Meningitis can be excluded by examination of the cerebrospinal fluid (CSF). Rabies should be considered when there are symptoms of brainstem dysfunction, including dysphagia and respiratory muscle dysfunction. A history of exposure to secretions of an infected animal is the most helpful historical point. In addition, rabies does not cause trismus. The four treatment strategies for patients with tetanus should be undertaken simultaneously: aggressive supportive care, elimination of unbound TS, active immunization, and prevention of further toxin production.25–27 Supportive Care.: Supportive care begins with control of the muscle spasms. Reflex spasms can result from stimulation of the patient, such as that caused by any movement of the patient or loud noises. Avoidance of unnecessary stimulation is recommended. Benzodiazepines are the mainstay of symptomatic therapy for tetanus. These drugs are GABA agonists and indirectly antagonize many of the effects of TS. They have no effect on the inhibition of glycine release by TS. Diazepam is the most extensively studied of these agents, but lorazepam and midazolam are equally effective. Diazepam has a rapid onset of action and a wide margin of safety, and it can be given orally, rectally, or intravenously. It is inexpensive and thus available in most parts of the world. It has a long cumulative half-life and active metabolites that can cause prolonged sedation and respiratory depression. The intravenous formulations of diazepam and lorazepam contain propylene glycol, which, at high doses, can produce lactic acidosis. Gastrointestinal delivery of these agents is limited by motility problems associated with tetanus. Midazolam has a short half-life and does not contain propylene glycol, but it should be given by continuous infusion and is cost-prohibitive in most areas of the world. Propofol infusion is effective, but it is also expensive, and patients may not tolerate the lipid vehicle. Neuroleptics, barbiturates, and intrathecal baclofen have no advantage over benzodiazepines. Dantrolene is a direct muscle relaxant without CNS activity. It has been reported as an adjunctive agent for muscle spasms and may decrease the need for mechanical ventilation.37 Magnesium sulfate infusion has been advocated as both adjuvant and first-line therapy for tetanus. Alone or in combination with other agents, it improves spasm control and may alleviate some of the autonomic instability associated with tetanus toxicity.38 If spasm cannot be controlled with these regimens or if any signs of airway compromise develop, the patient should receive neuromuscular blockade and mechanical ventilation. Although succinylcholine can be used in the initial phase of the disease, the clinician should be aware of the risk of severe hyperkalemia resulting from its use in any neuromuscular disease. This effect does not begin until about 4 days after the onset of disease.25 Long-acting nondepolarizing agents are preferred, even in the initial phase. Pancuronium has traditionally been used, but it is an inhibitor of catecholamine reuptake and may worsen autonomic instability.25 Vecuronium and rocuronium are shorter acting and are without significant cardiovascular side effects but require continuous infusion. Whichever agent is used, adequate sedation should be provided, and neuromuscular blockade should be withheld at least once a day to assess the patient’s status. All intubated patients should be considered for early tracheostomy to decrease reflex spasms caused by the endotracheal tube.32 Autonomic instability requires monitoring and aggressive treatment. Sympathetic hyperactivity can be treated with combined alpha- and beta-adrenergic antagonists, such as labetalol and propranolol. The use of beta-antagonists alone can lead to unopposed alpha-activity, resulting in severe hypertension.34 If beta-antagonists are necessary, a short-acting agent such as esmolol should be used.26 Because the episodes of the autonomic crises in tetanus are due to catecholamine excess, the use of pure beta-antagonism can result in unopposed alpha-agonism. Clonidine has shown variable success at modulation of sympathetic outflow in these cases.34 Morphine and magnesium sulfate infusions as well as spinal anesthesia and intrathecal baclofen have been shown to improve autonomic dysfunction.25 Diuretics should be avoided for blood pressure control as volume depletion can worsen autonomic instability. Bradydysrhythmia should be treated with temporary pacing. Atropine and sympathomimetic drugs should be used with caution as the autonomic instability is essentially due to catecholamine excess.26,27,34 Elimination of Unbound Tetanospasmin and Active Immunization.: Human tetanus immune globulin (HTIG) and Td should be administered as soon as possible to all patients with suspected tetanus. Tetanus immune globulin (TIG) does not neutralize toxin already present in the nervous system, nor does it treat any existing symptoms. HTIG neutralizes any circulating toxin as well as toxin at the site of production and reduces mortality. TIG should be administered at a site separate from the toxoid. Dosage recommendations vary (500-10,000 units of TIG), but multiple injections are stimuli for spasm, and most authorities note that 500 units is as effective as higher doses. Adult and pediatric doses are the same. If the larger doses are used, they should be given in divided injections. Administration of a portion of the TIG proximal to the site of inoculation is often recommended but has not been studied.25–27 Protective antibody levels are achieved 48 to 72 hours after administration of TIG. Because the half-life of TIG is 25 days, repeated doses are not needed. The preparation of TIG available in the United States is not licensed for intrathecal administration, which is of no proven benefit.25,27,32
Bacteria
Diphtheria
Background
Epidemiology
Principles of Disease
Pathophysiology
Clinical Features
Complications
Diagnostic Strategies
Differential Considerations
Management
Pertussis
Background
Epidemiology
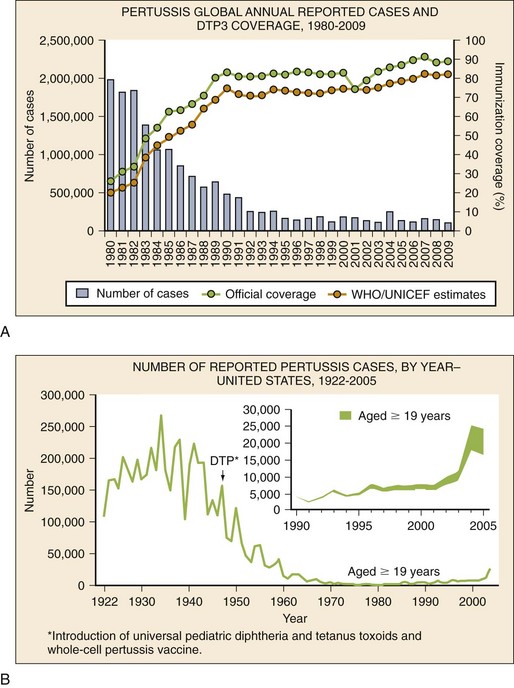
(From World Health Organization, Department of Immunization, Vaccines and Biologicals: WHO Vaccine-Preventable Diseases: Monitoring System—2010 Global Summary. Geneva, World Health Organization, 2010.)
B, Incidence of reported pertussis cases in the United States by year.
(From Centers for Disease Control and Prevention National Notifiable Diseases Surveillance System. Reproduced from MMWR Recomm Rep 55:1-33, 2006.)
Principles of Disease
Pathophysiology
Clinical Features
Complications
Diagnostic Strategies
Management
Vaccination
Tetanus
Background
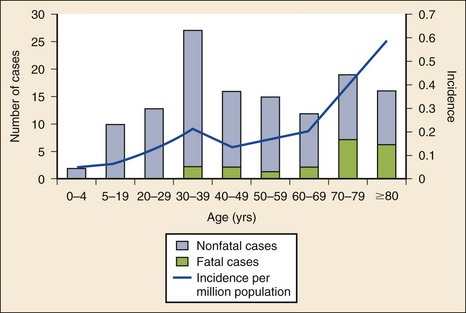
Epidemiology
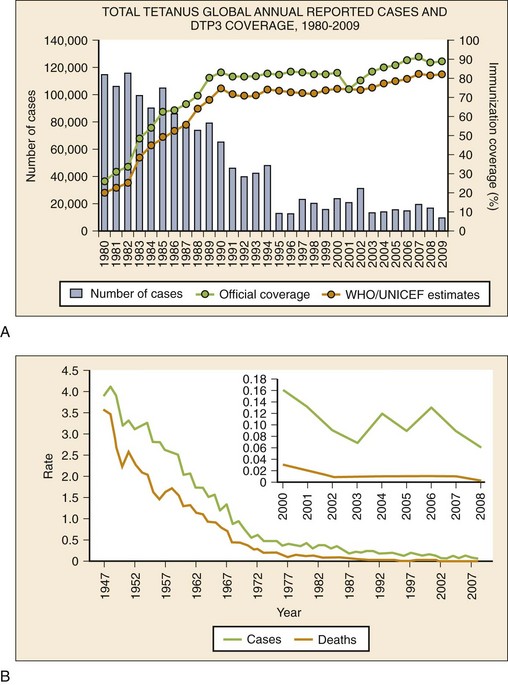
(From World Health Organization, Department of Immunization, Vaccines and Biologicals: WHO Vaccine-Preventable Diseases: Monitoring System—2010 Global Summary. Geneva, World Health Organization, 2010.)
B, Incidence of reported tetanus cases in the United States by year. (From Centers for Disease Control and Prevention National Notifiable Diseases Surveillance System. Reproduced from MMWR Morb Mortal Wkly Rep 60:365-369, 2011.)
Etiology
Principles of Disease
Clinical Features
Complications
Diagnostic Strategies
Differential Considerations
Management

Full access? Get Clinical Tree


Bacteria
Only gold members can continue reading. Log In or Register to continue