CHAPTER 69
Asthma
Jose B. Contreras, MD • Afnan Tariq, MD • Ricardo Gomez, MD • Manhal J. Izzy, MD • Jean G. Ford, MD
Asthma is one of the most common problems in pediatric and adult primary care. Poorly controlled asthma is a frequent cause of unscheduled medical visits, including emergency department visits. It is also a major cause of preventable hospital admissions for both children and adults. However, there have been major advances in the understanding of the pathobiology of asthma, and a substantial body of evidence has accrued over the past 25 years, on new treatment modalities for asthma. If this knowledge is applied in primary care practice, it will be possible to reduce the incidence of failure of primary care management for asthma, as indicated by emergency department visits and hospitalization. Moreover, application of this knowledge would substantially increase the proportion of people who achieve an excellent quality of life while living with asthma.
This chapter reviews the pathophysiology of asthma, and discuss its management in the context of primary care.
 ANATOMY, PHYSIOLOGY, AND PATHOLOGY
ANATOMY, PHYSIOLOGY, AND PATHOLOGY
Asthma is a chronic disorder of the airways. It is characterized by inflammation, airflow obstruction, and bronchial hyperresponsiveness. Its symptoms are variable and recurring, and multiple factors influence its clinical manifestations, severity, and response to treatment. Mechanisms of airflow obstruction in asthma include (1) bronchoconstriction, that is, quick narrowing of the airways secondary to contraction of bronchial smooth muscle in response to allergens or irritants; (2) bronchial hyperresponsiveness, that is, exaggerated bronchoconstriction in response to airway stimuli (e.g., environmental tobacco smoke); and (3) airway edema, a manifestation of persistent and progressive inflammation, which may be accompanied by mucus hypersecretion and mucus plugs (National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma [EPR3], 2007).
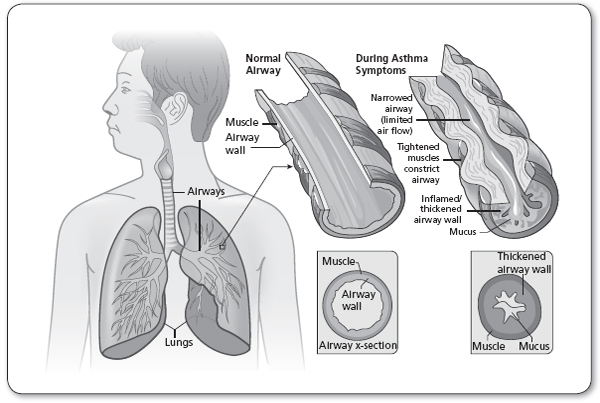
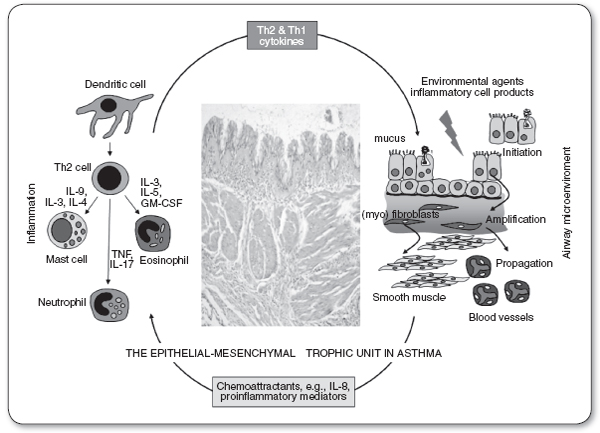
Beginning at the level of the trachea and the main stem bronchi, the human airway divides into ever-narrowing and smaller branches. The trachea and main bronchi are surrounded by nonstriated muscles and fibrous tissue, and lined with ciliated epithelial cells, which remove mucus produced by goblet cells neutrophils and lymphocytes (Figure 69.1). The chronic airway inflammation in asthma involves many different types of cells, including mast cells, the most important inflammatory cells, eosinophils, neutrophils, T lymphocytes, macrophages, and epithelial cells (Figure 69.2). This inflammation causes recurrent episodes of coughing (usually worse at night), wheezing, chest tightness, and difficulty breathing. Such episodes are associated with reversible airflow obstruction, mediated principally by airway inflammation, and also increased bronchial smooth muscle contraction. With chronic, persistent inflammation, and recurrent attacks, there may be evidence of extensive epithelial cell damage, loss of ciliated mucosa and basement membrane thickening, as well as goblet cell hyperplasia, along bronchial wall edema. Hyperplasia of the bronchial smooth muscle cells, mucus hypersecretion, and angiogenesis are also common in this context. All these chronic changes thicken the airway walls and narrow the airway diameter, reflecting airway remodeling. The patient, instead of having only episodic symptoms, could become constantly symptomatic, as in other obstructive lung diseases, with incomplete reversibility of the airway obstruction (Hirota & Martin, 2013).
The patterns of inflammatory response in asthma (intensity, cellular mediators, and therapeutic response) may correlate with specific asthma manifestations. For example, Th2-mediated cytokine responses to aeroallergen are common in asthma; however, neutrophilic inflammation is relatively more common in sudden-onset, fatal asthma exacerbations, in occupational asthma, and among asthmatic patients who smoke.
There is increasing evidence of the importance of gene–environment interactions in asthma (Kauffmann & Demenais, 2012). Atopy, the genetic predisposition for the development of an immunoglobulin E (IgE)-mediated response to common aeroallergens, is the strongest identillable predisposing factor for developing asthma. Viral respiratory infections are one of the most important causes of asthma exacerbation and may also contribute to the development of asthma. Cholinergic stimulation causes constriction of the bronchi, dilation of the local blood vessels, and increased mucus production. Adrenergic stimulation leads to bronchodilation, mucosal vasoconstriction, and increased ion secretion, resulting in increased ciliary rate and mucus clearance. The respiratory epithelium has an extensive network of beta-2 receptors that oppose bronchoconstriction. Stimulation of the beta receptors on the mast cells and other inflammatory cells stabilizes them and inhibits the release of inflammatory mediators. Bronchodilation results from circulating catecholamines, such as epinephrine, and glucocorticoids, which are affected by circadian rhythms, resulting in lower levels at night and early morning, and a concomitant increased risk of asthma symptoms.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Asthma is one of the most common diagnoses in both adult and pediatric primary care. The burden of asthma in the United States grew steadily, between 1980 and 2010. It is estimated that 25.9 million people in the United States have asthma (Schiller, Lucas, & Peregoy, 2012), including 18.9 million adults. Approximately 8.2% of adults, and 9.5% or 7.1 million children, have asthma (Summary Health Statistics for U.S. Children; National Health Interview Survey, 2011). Asthma is the cause for 15 million primary care office and hospital clinic visits, and 2 million emergency department visits per year (National Ambulatory Medical Care Survey: 2010 Summary Tables). It is also the primary diagnosis for approximately 479,000 hospitalizations per year, with an average length of stay of 4.3 days; and it was the primary cause of death for 3,404 Americans in 2010. Asthma exacerbations are frequent, as 53% of individuals with asthma reported an attack in 2008, including 57% of children with asthma (Centers for Disease Control and Prevention, 2011). The prevalence of asthma is higher among women than men, and among boys than girls. The disease has a disproportionate effect on non-Hispanic Blacks, American Indian, and Puerto Ricans: 11% of non-Hispanic Blacks of all ages and 17% of non-Hispanic Black children had asthma in 2009, the highest prevalence among racial/ethnic groups. From 2001 to 2009, asthma rates rose nearly 50% among Black children, the highest rate increase. From 2008 to 2010, prevalence was highest among self-reported multiracial, Black, and American Indian persons.
Low-income people, irrespective of race or ethnicity, have a disproportionate burden of asthma. Despite the higher burden of disease among low income and racial and ethnic minority populations, access to and the quality of medical care or asthma provided to those populations is often poorer, compared to their counterparts (National Hospital Ambulatory Medical Care Survey, 2010a). Moreover, the higher prevalence of exposure to asthma triggers in socioeconomically disadvantaged populations suggests the need for special efforts, in order to deliver primary care for asthma, consistent with current guidelines (National Hospital Ambulatory Medical Care Survey, 2010b).
 DIAGNOSTIC CRITERIA
DIAGNOSTIC CRITERIA
Patients usually present with a history of nonspecific symptoms, including dyspnea, cough, and/or wheezing, the classic symptoms of asthma. They may also present with chest tightness or difficulty breathing at night. The symptoms are worse at night and in the early morning, and cough is sometimes the only reported symptom. Compared with unaffected individuals adult asthma patients are more likely to have experienced respiratory symptoms during childhood, reflecting undiagnosed childhood asthma (Yunginger et al., 1992). Common triggers of asthma symptoms include exercise, airborne allergens or irritants, weather changes, viral infections, stress, strong emotions, seasonal changes, and menstrual cycles (Table 69.1).
Factors and Conditions Known to Trigger an Asthma Attack |
Exercise
Drugs (aspirin/NSAIDs)
Irritants (sulfur dioxide and formaldehyde)
Food additives/dairy products
Pregnancy
GERD
Cigarette smoking, including secondhand smoke
Household factors (damp house, gas cooking, humidifiers)
Indoor environmental hazards (silica, chemical fragrances)
GERD, gastroesophageal reflux disease; NSAIDs, nonsteroidal anti-inflammatory drugs.
The symptoms are episodic. Certain chronic occupational exposures can result in airway sensitization, ultimately manifesting as occupational asthma, which is characterized by asthma symptoms that worsen in association with workplace exposures (see Chapter 73 on Occupational Lung Disease). There is an increased likelihood of asthma among patients with a family history of atopy. The clinical diagnosis of asthma does not require laboratory testing; however, in some instances, a positive skin test to triggering allergens and an elevated serum IgE could support the diagnosis.
A variety of psychosocial factors influence asthma outcomes (Weil et al., 1999). Alcohol abuse, recent unemployment, and measures of social instability have been associated with an increase risk of asthma death. Asthma is generally more difficult to control among patients with mental illnesses, such as depression and schizophrenia (Van Lieshout & MacQueen, 2008). The experience of uncontrolled asthma has been found to be associated with lower self-esteem (McNelis et al., 2000), higher stress levels. This may be related, to some extent to the patient’s social support systems, including the response of family and friends to the disease, as well as the patient’s approach to coping with emotional stress (McCormick et al., 2014).
 HISTORY AND PHYSICAL EXAMINATION
HISTORY AND PHYSICAL EXAMINATION
The physical examination should help determine whether the patient needs an immediate intervention. Major areas of focus include the airway, chest wall, breath sounds, and the skin. The vital signs should be reviewed for the respiratory rate, pulse, and whether there is pulsus paradoxus. Inspection of the chest wall can help determine whether there is hyperinflation. Chest auscultation can help identify stridor, wheezing or rales, and prolongation of the respiratory rate. It should be noted that wheezing can be absent, even in the context of significant airway narrowing.
Indicators of a severe asthma episode include the inability to speak in full sentences, tachycardia, tachypnea, nasal flaring, and intercostal retractions. These signs should alert the clinician of the potential need for emergent action, including intubation. The physical examination may be normal in the presence of asthma. The skin examination should help identify signs of eczema on flexor surfaces. The presence of other signs of atopy (rhinitis, nasal polyps) should also be noted (Figure 69.4).
 DIAGNOSTIC STUDIES
DIAGNOSTIC STUDIES
In addition to the history and physical examination, it is critical to obtain objective measures in support of the diagnosis of asthma. When considering the diagnosis of asthma, baseline spirometry with bronchodilators should be conducted, to assess airway patency, and determine whether airflow obstruction, if present, is reversible. In spirometry, the patient conducts a maximal inhalation, followed by a forceful and rapid, complete exhalation. Practitioners can use the forced vital capacity (FVC) and the forced expiratory volume in 1 second (FEV1) to determine (a) whether there is airway obstruction, as indicated by an FEV1/FVC ratio of <0.75; (b) the extent of airflow limitation; and (c) following the administration of beta2-agonist, whether there is reversibility, that is, an increase in FEV1 of 12% and 200 mL, compared to the baseline FEV1 (American Thoracic Society, 1991). The flow-volume loop, obtained at spirometry, should be reviewed to detect any evidence of upper airway disorders that mimic asthma.
Asthma patients can have normal spirometry results. When the diagnosis is suspected despite normal spirometry with bronchodilators, bronchoprovocation testing, usually with methacholine, can be considered, as a means for demonstrating bronchial hyperactivity. A bronchial provocation test can be done using methacholine or histamine. This type of testing is useful for diagnosing asthma in patients with normal baseline airflow. The drugs are delivered to the patient from a nebulizer and the concentration is doubled until there is a 20% decrease in the initial FEV1. This concentration is known as the PC20. The test is considered positive when a provocative concentration of 8 mg/mL or less causes the decrease of 20% of the FEV1. An FEV1 <80% of the FEV1 before administration is diagnostic of bronchial hyperactivity. An occupational challenge may be considered, based on the history of workplace exposures.
Serial peak expiratory flow (PEF) readings, obtained from a brief, forceful exhalation, may support the diagnosis of asthma. The PEF can also be used to monitor asthma control. The patient and the health care provider should know the personal best PEF, as reduced PEF levels are a common feature of worsening asthma.
Imaging and Blood
While not essential to the diagnosis of asthma, a chest x-ray can be used to exclude causes of airway narrowing other than asthma. When patients use medications regularly for sinusitis, but without improvement, sinus x-rays or CT scan can be used to exclude an alternative diagnosis. Similarly, an evaluation for gastroesophageal reflux is also important in the diagnostic evaluation. Eosinophilia in sputum or nasal secretions is characteristic of asthma, but neutrophils are more common in bronchitis.
 TREATMENT OPTIONS, EXPECTED OUTCOMES, AND COMPREHENSIVE MANAGEMENT
TREATMENT OPTIONS, EXPECTED OUTCOMES, AND COMPREHENSIVE MANAGEMENT
Classification of Asthma and Severity of Disease
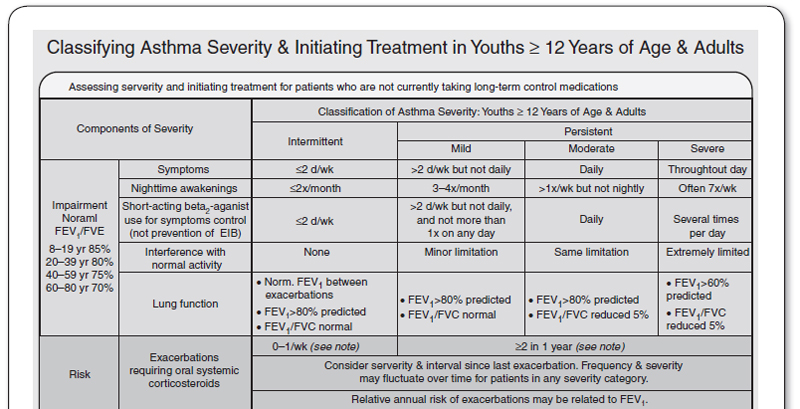
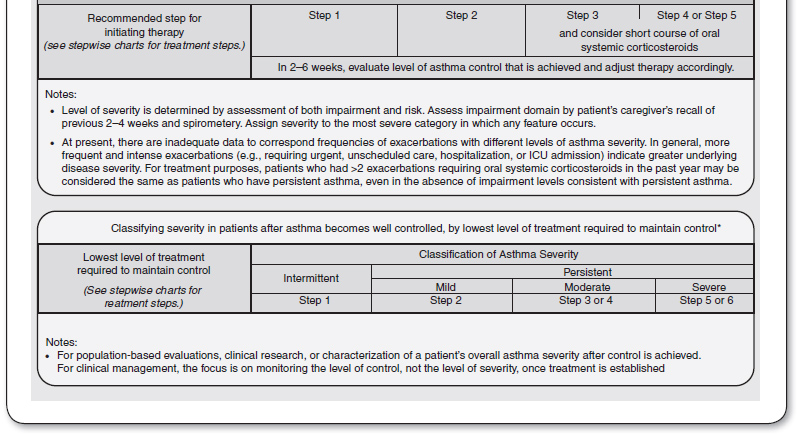
It is critical to classify asthma severity, because it is the basis of effective therapy. Symptoms and lung function are used to classify asthma severity (Figure 69.3). Patients can record their symptom in a diary, and monitor their lung function with a peak flow meter. For monitoring purposes, PEF should generally be measured within 15 minutes of waking up in the morning, before the use of a bronchodilator. Specific indicators of severity are: the frequency of symptoms per week, nocturnal awakenings, rescue bronchodilator use, effects of asthma symptoms on daily activities, and the frequency of exacerbations resulting in the use of systemic corticosteroids (oral or IV; see Figure 69.1). When the PEF drops to <80 % of the personal best, this is a sign of impending asthma attack. The patient should be instructed to alert the primary care physician when the PEF drops below 80% of their personal best; they should seek emergency care if it drops to 50% or less.
FIGURE 69.3
Classifying asthma severity and initiating treatment in adults and adolescents.
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Pharmacotherapy of Asthma
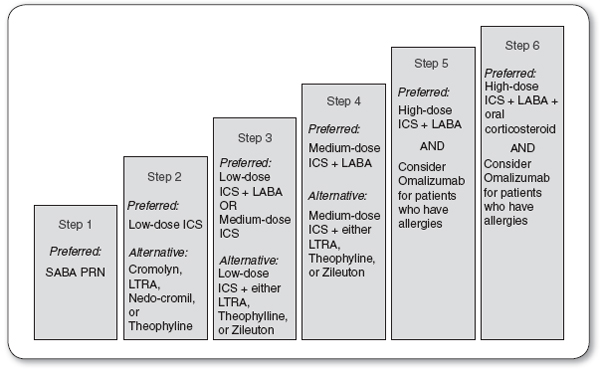
Asthma is medically managed with a combination of quick relief and long-term controller medications, in a step-wise approach (Figure 69.4).
FIGURE 69.4
Stepwise approach for long-term asthma management.
ICS, inhaled corticosteroids; LABA, long-acting beta2-agonists; LTRA, leukotriene receptor antagonists; PRN, as needed; SABA, short-acting beta2-agonists.
QUICK RELIEF
Short-acting beta2-agonists (SABAs) are used for immediate relief from acute symptoms, including wheezing, cough, and chest tightness. SABAs are the drugs of choice for treating exacerbations, acute asthma symptoms, and exercise-induced bronchospasm. They cause airway smooth muscle relaxation, thereby causing bronchodilation within 3 minutes. The use of levalbuterol, a stereoselective SABA that theoretically causes less tachycardia than albuterol, has grown significantly during recent years. However, there is no evidence that levalbuterol is more effective than albuterol at providing immediate relief of symptoms. Regular use of daily SABAs is not recommended, as it does not yield additional benefits, in comparison to as-needed-only treatment. When patients use SABAs more than two times per week, this is a sign that their asthma symptoms are not controlled, and it suggests the need for anti-inflammatory medications.
During emergency department visits for asthma, anticholinergic medications may be used, along with SABAs, to treat moderate to severe acute exacerbations. It should be noted, however, that the bronchodilator effects of ipratropium bromide have a slower onset than those of albuterol, and it has not been shown, to date, that the addition of ipratropium, improves outcomes of acute asthma exacerbations, compared to albuterol alone. Moreover, is not recommended for routine use in outpatient asthma management.
Long-Term Controller Medications
Long-term controller medications are indicated for patients with persistent symptoms, in order to reduce their dependence on quick relief. Except for long-acting beta2-agonists (LABAs), most of the long-term controller medications have anti-inflammatory properties (National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma [EPR3], 2007). LABAs cause sustained bronchodilation, thereby potentially reducing the need for quick-relief medications.
![]() CLINICAL WARNING:
CLINICAL WARNING: