Fig. 7.1
Pressure support ventilation (PSV) algorithm: each patient’s effore troggers the ventilator (yellow arrows). The ventilator assists the spontaneous inspiratory effort with a pre-set constant level of pressure (dotted red line). The interplay between the ventilator assistance and the spontaneous effort generates an ispiratory flow peak (dotted red circles) that ts different on a breth by breath basis. Once the inspiratory flow decays below a prefixed threshold (expiratory trigger, blue arrows) the ventilator cycles off into the expiratory phase
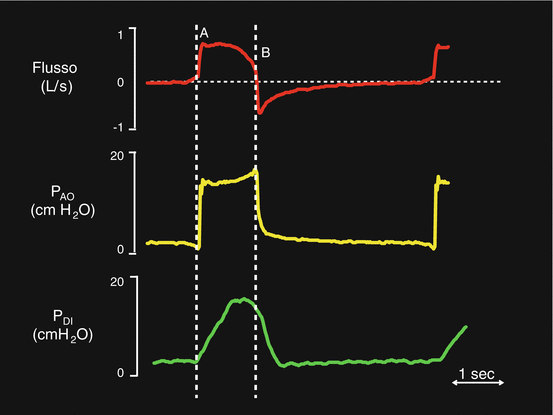
Fig. 7.2
Flow (red trace), airway opening pressure (PAO– yellow trace) and transdiaphragmatic pressure (PDI– green trace) during PSV. The dotted white line A indicates the inspiratory trigger phase. The dotted white line B indicates the expiratory trigger phase
Generally, during PSV, the breaths are flow triggered but the pressure trigger mode can be used too.
With the pressure trigger, the ventilator starts to deliver the assistance when the patient’s spontaneous respiratory effort generates a negative pressure inside the ventilator circuit. Instead, if the flow trigger is used, the assistance starts when the patient subtracts a predefined portion of a continuous gas flow (“bias” flow) that circulates in the circuit at end expiration. Any delay in the delivery of the assistance is named “inspiratory trigger delay”. The amount of delay is greatly influenced by the technical features of the single ventilator but depends also on several clinical parameters, mainly patient’s breathing pattern and the presence of dynamic hyperinflation [13].
The positive pressure applied by the ventilator to assist the spontaneous inspiratory effort is constant throughout the whole pressurization phase. During this phase, the ventilator maintains the preset level of positive pressure at the airway opening by replacing (virtually in real time) moment by moment the volume delivered to the patient. In most ventilators it is possible to adjust the pressurization rate (the time needed to reach the pressure support level, i.e. the slope of pressurization). In the common practice, the pressurization slope is set at 0.1–0.2 s. A peak and a subsequent approximately exponential decay characterize the inspiratory flow profile. The peak flow is greatly influenced by the slope of pressurization and by the early inspiratory effort. The subsequent flow rate decay depends on the interplay between the inspiratory efforts, the mechanical properties of the respiratory system and the amount of volume delivered to the patient. Ideally, until the inspiratory effort is active, after the inspiratory flow peak there will be a sustained, slowly decaying flow. When the inspiratory effort comes to an end, the inspiratory flow sharply decreases. The expiratory trigger is generally activated when the inspiratory flow decays below a given threshold, suggesting that the patient is no longer active in generating the inspiratory effort. Usually the expiratory trigger threshold is not fixed but is a percentage of the previous flow peak. For example, if the threshold is 30% of the inspiratory flow peak rate, the cycle will occur at 0.3 L/min if the peak flow is 1 L/min. In most ventilators, the expiratory trigger threshold is adjustable from 70 to 10% of the flow peak.
Figure 7.1 shows a typical ventilator screen during PSV. We can see pressure (PAO) and flow curves. The yellow arrows indicate the inspiratory trigger activation. The red dotted line represents the pressure support level. Note that the inspiratory time changes breath by breath (red arrows). The flow peak is also variable as indicated by red dotted cycles. The blue arrows indicate the expiratory trigger activation.
Figure 7.2 illustrates an ideal PSV breath. The diaphragmatic electrical activity (EAdi) trace (green line) is also visible. In this ideal situation, the diaphragm contraction activates the inspiratory trigger and the pressurization phase starts (PAO-yellow trace). Letter A indicates the flow peak. At the end of diaphragmatic contraction, the flow reduces and, at last, it decays to the expiratory trigger threshold.
In clinical practice, the level of support is titrated to obtain a VT between 5 and 8 ml/predicted body weight (PBW) and a RR between 15 and 30 breaths/min [9, 14]. That’s why, according to physiological studies, a VT higher than 8 ml/PBW and a RR lower than 15 bpm are generally signs of over-assistance while, on the contrary, a VT lower than 5 ml/PBW and a RR higher than 30 bpm are signs of under-assistance [15].
Several physiological studies demonstrated the PSV ability, as compared with controlled mechanical ventilation, to reduce the adverse effects of prolonged sedation and ventilator-induced diaphragm dysfunction [5, 7, 9, 15]. Despite these positive reports, however, poor patient-ventilator interactions frequently occur during PSV. To understand why this occurs, it is convenient to introduce the “neuro-ventilatory coupling” concept [10] (Fig. 7.3). In healthy subjects, small changes in the respiratory effort determine high variations of flow and VT, and the physiological effort is about the 20–30% of the maximum inspiratory capacity (green line, Fig. 7.3) [16]. Whenever the neuro-ventilatory coupling is impaired, the slope and the variability of the neural output/VT or neural output/inspiratory flow relationship decrease (red line, Fig. 7.3). Considering a patient with an impaired neuro-ventilatory coupling ventilated in PSV, the slope of the neuro-ventilatory coupling remains a pathological one, but the inspiratory assistance provides a bust that translates each point of the line toward a higher VT and inspiratory flow. Accordingly there will be only one point (red point, Fig. 7.3), where the neuro-ventilatory coupling is “normal”. This may explain why the over-assistance phenomenon frequently occurs during PSV. Over-assistance may reduce assisted mechanical ventilation advantages and side effects, generally associated with controlled mechanical ventilation, may prevail.
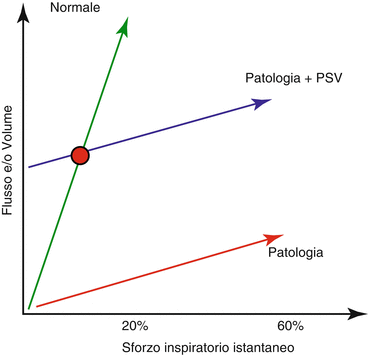

Fig. 7.3
Neuro-ventilatory coupling. Neuro-ventilatory coupling relationship in healty subjects (green line), pathological subjects (red line), pathological subjects during PSV (blue line). The red point figures out the only one point in which physiological neuro-ventilatory coupling, during PSV, is preserved
Over-assistance may frequently occur during PSV. Since the level of assistance is fixed and doesn’t change with the patient’s spontaneous effort, if the patient effort is weak and ceases very early, the ventilator inflates the passive patient up to the expiratory trigger threshold. Accordingly, the combination of high levels of PSV, weak and short inspiratory effort and low expiratory trigger threshold easily generates over-assistance during PSV. If the patient just triggers the ventilator and immediately ceases the inspiratory effort, the assistance delivery will be independent by the patient effort, and VT depends entirely on the level of assistance and the mechanical properties of the respiratory system. During over-assistance, the inspiratory mechanical time (Timech) is longer than the neural inspiratory time (Tineur).
In most cases, as said above, when a patient is over-assisted, the VT is higher than 8–10 ml/Kg predicted body weight (PBW), and/or the RR is lower than 15 breaths/min. However, recent studies point out that, interestingly, over-assistance may occur even if RR and VT are in the suggested clinical range. Figure 7.5, adapted from ref. [17], shows the VT, RR and diaphragmatic WOB trend in 12 patients ventilated in PSV for 48 h [17]. Note that the diaphragmatic WOB was constantly under its physiological range throughout the period despite the PSV settings were in line with the clinical best practice, i.e. the PSV level was titrated to obtain a VT between 5 and 8 ml/PBW and RR between 15 and 30 breaths/min. Our group recently recorded the diaphragmatic electrical activity (EAdi) during prolonged PSV (12 h), in 17 patients (unpublished data). The EAdi represents the neural ventilator output and is strictly related with diaphragmatic WOB; we predefined four EAdi categories:
NO EAdi: EAdi absent (the patient starts the breath with the accessory muscles and is subsequently over-assisted by the ventilator)
LOW EAdi: EAdi under 5 μV
NORMAL or MEDIUM: EAdi in the normal range of 5–15 μV
HIGH: EAdi above 15 μV
The results are shown in Fig. 7.4. The NO EAdi condition occurred the 20.9% of the total recorded patients’ breaths. The LOW EAdi condition occurred in the 52.8% of the total breaths and, finally the NORMAL and the HIGH EAdi conditions occurred in the 20.7 and the 5.6%, respectively.
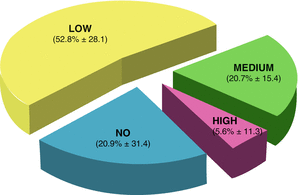

Fig. 7.4
Percentage of Eadi cathegories in 17 patients during prolonged PSV (12 hours). Please note the prevalence of NO-EAdi and LOW-Eadi conditions
Figure 7.5 illustrates PAO, flow and EAdi traces in one representative patient. Note that the EAdi decreased during the 12 h, whereas the flow and the PAO curves didn’t change significantly.
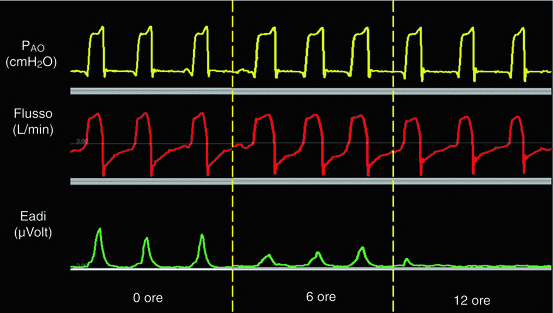

Fig. 7.5
PAO (yellow trace), Flow (red trace) and Eadi (green trace) in one representive patient during the 12 hours of PSV. Eadi significantly decreased throughout the hours whether PAO and flow traces remained unchanged
Over-assistance is one of the most important causes of patient-ventilator asynchrony [18]. Several clinical trials have demonstrated that a high incidence of patient-ventilator asynchronies is correlated with a longer ICU length of stay and mechanical ventilation duration, a higher incidence of tracheostomy and, at last, a higher mortality [19–21].
Figure 7.6 shows the relationship between mechanical respiratory rate (RRmech) and spontaneous “neural” respiratory rate (RRneur) in 20 patients ventilated in PSV for 8 h (unpublished data). In a group of patients, that were mainly patients with moderate-severe COPD, the figure shows a severe discrepancy between RRmech and RRneural. Figure 7.7 shows the flow, the volume, the PAO and the oesophageal pressure (Pes) traces in one representative patient ventilated in PSV for 8 h. Each panel represents 30 s taken at the beginning of each hour of the study. In this patient, patient-ventilator interactions remarkably varied throughout the study period. Asynchronies were evident at 3, 4 and 7 h.
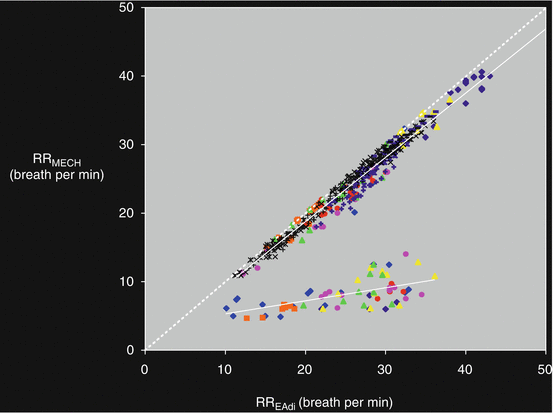
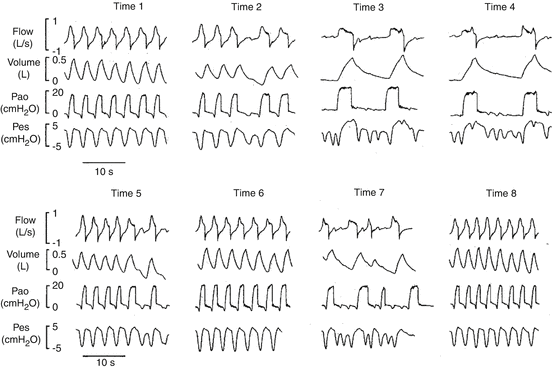

Fig. 7.6
Relationship between mechanical respiratory rate (RRmech) and spontaneous “neural” respiratory rate (RRneural) in 20 patients ventilated in PSV mode for 8 hours. The figure illustrates the discrepancy between RRmech and RRneural in some of the studied patients

Fig. 7.7
Flow, volume, PAO and esophageal pressure (Pes) in one representive patient during the 8 hours of PSV. We can see the changes in terms of patient-ventilator interactions throughout the study. Please note the high percentage of asynchronies at 3, 4, and 7 hours
Prolonging mechanical insufflation into neural expiration has been shown to worsen dynamic hyperinflation and cause ineffective inspiratory efforts. This may happen principally in COPD patients who need a higher respiratory effort to overcome the intrinsic PEEP (PEEPi) [22].
A mean to improve the patient-ventilator interactions during PSV is to titrate the slope of pressurization, the level of assistance and the expiratory trigger threshold in order to optimize patient-ventilator interactions, assure the optimal diaphragmatic workload and minimize the asynchronies [20]. Generally speaking, the peak inspiratory flow increases with the slope of pressurization and vice versa, and, thus, the higher is the peak inspiratory flow the higher will be the mechanical inspiratory time (because the expiratory trigger threshold is a percentage of the peak inspiratory flow). On the other hand, the expiratory trigger threshold has a deep influence on the inspiratory time (the lower the threshold, the higher the mechanical inspiratory time). Titrating the assistance level could serve to circumvent over- and under-assistance. However, the PSV critically depends from the single clinicians’ expertise, and one could consider it more an art than a science. The fact that during PSV the over-assistance may occur even if RR and VT are in the “optimal” range (see above) makes it difficult to trust solely on the flow and PAO traces to titrate the PSV level, the slope of pressurization and the expiratory trigger threshold. Experts are concordant in suggesting bedside monitoring of respiratory muscle activity to easily detect asynchronies and to avoid over- or under-assistance, but on the other hand, reliable indexes of diaphragmatic and intercostal muscle activity to be used at the bedside are scanty. The recent introduction in clinical practice of the EAdi monitoring (see below) and of diaphragmatic electromyography could represent a turning point to monitor diaphragm activity bedside on a breath-by-breath basis [23, 24].
7.3.1 EAdi and NAVA
During NAVA, the ventilator assistance is proportional to the patient’s spontaneous diaphragmatic activity (EAdi). The diaphragmatic electromyography strictly correlated with phrenic nerve discharge [12, 25] and hence to the neuro-ventilatory drive. From a technical point of view, the EAdi is measured through an array of eight electrodes mounted within a nasogastric tube (NAVA catheter) (Fig. 7.8). The EAdi signal is obtained from the crural portion of the diaphragm, amplified and filtered from cardiac artefacts and other electrical contaminations.
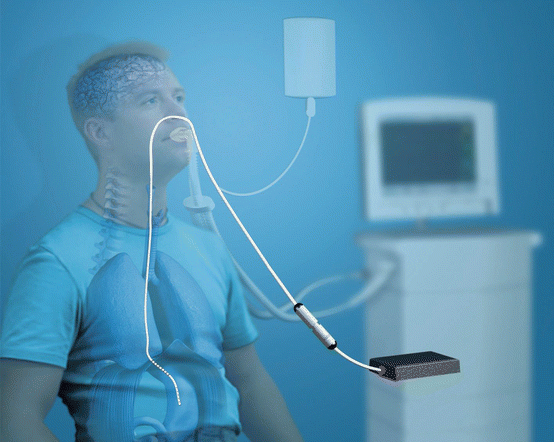

Fig. 7.8
Eadi signal acquisition during NAVA. Eadi is measured through a naso-gastric tube equipped with 8 electrodes (NAVA catheter) and is processed and visualized on the ventilator screen
Besides monitoring the neuro-ventilatory drive, the EAdi signal can be used to evaluate the diaphragmatic efficiency in terms of neuromechanical and neuro-ventilatory efficiency (NME and NVE, respectively) [23, 26]. The NME is calculated as the ratio between the negative pressure developed by the diaphragm and the EAdi peak during an end-expiratory occlusion and is expressed in cmH2O/ μV. The neuro-ventilatory efficiency, expressed in ml/ μV, is calculated as the ratio between VT and the correspondent EAdi peak. Figure 7.9 shows how to calculate these two parameters. Bellani and co-workers validated a technique to continuously calculate the diaphragmatic WOB from NME and the EAdi signal [27]. Recently Liu and coll [26] demonstrated that the NME and NVE evaluation was useful to predict extubation readiness.
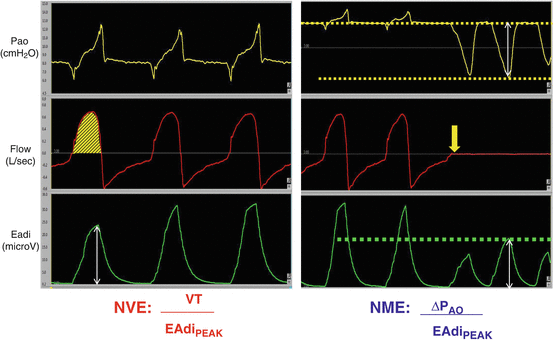

Fig. 7.9



Neuro-ventilatory efficiency (NVE, panel on the left) and neuro-mechanical efficiency (NME, panel on the right) calculation. The NVE is the ratio between tidal volume (VT, yellow area under the flow trace, red trace) and the Eadi peak (white arrow under the green trace). The NME is the ratio between negative pressure during an end-expriratory occlusion (yellow trace) and Eadi peak (green trace). During the expriratory occlusion the flow is zero (red trace)

Full access? Get Clinical Tree




