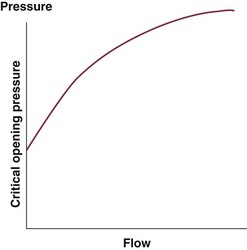
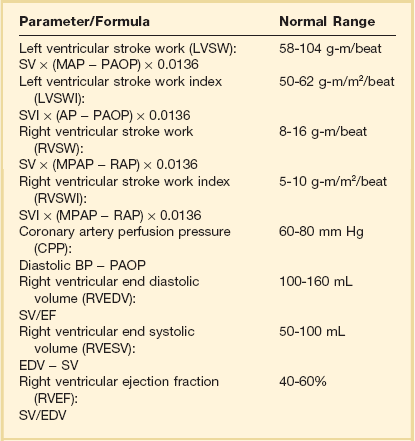
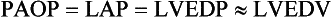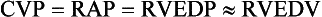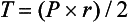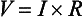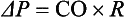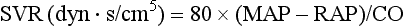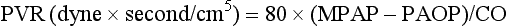3 The accurate assessment and manipulation of the circulation are important parts of the management of critically ill patients. The principal objective of cardiorespiratory manipulation is to ensure optimal oxygen delivery. Adequate cellular respiration, adequate delivery of substrates and pharmaceuticals, and eventual recovery of organs and tissues are possible only when this has been achieved. This chapter deals with the physiologic principles governing the cardiovascular system and discusses interpretation of hemodynamic data in a clinical context. With the increasing number of medical devices available to monitor the circulation, this chapter also outlines the principles underlying the design and function of these devices, as well as their uses and limitations (Tables 3.1 to 3.4). Table 3.1 Primary Measured Hemodynamic Data Table 3.2 Table 3.3 Our current understanding of the relationship between preload and contractility and their effect on stroke volume is based on the experiments of Dario Maestrini, Otto Frank, and Ernest Starling. Culminating in Starling’s “law of the heart,” which states that the force of myocardial contraction is directly proportional to the end-diastolic myocardial fiber length (or “preload”), as determined by the ventricular end-diastolic volume (EDV).1,2 It is this relationship, commonly known as the Frank-Starling mechanism that matches venous return to cardiac output (Fig. 3.1). The most common intervention used by clinicians to improve stroke volume—the administration of fluids—is aimed at increasing venous return as a main determinant of EDV. In this regard two factors are important to consider: the position of an individual heart on the Frank-Starling curve and the expected response to fluid administration, as well as giving the right amount of fluid in order to achieve a hemodynamic response. The dynamics of venous return have been extensively described by Arthur Guyton, whose theories contribute to our current understanding of the circulation and may help to optimize venous return.3,4 Preload is determined by the following factors: • Venous capacitance (i.e., sympathetic stimulation resulting in venoconstriction, mechanical compression) • Posture: The Trendelenburg position (supine, head down) increases venous return. Clinically, a passive leg raise test may be performed to assess volume responsiveness by autotransfusion of approximately 300 mL of blood from the splanchnic and lower limb compartments to the central circulation, increasing preload. • Intracavitary pressures: Abdominal hypertension and increased intrathoracic pressures (i.e., during positive-pressure ventilation and application of positive end-expiratory pressure [PEEP]) can oppose venous return. • Ventricular compliance: Ventricular compliance determines the relationship between EDV and end-diastolic pressure (i.e., diastolic dysfunction or impaired relaxation as in ventricular hypertrophy). • Heart rate: The filling time influences EDV. • Atrial contraction: Atrial contraction contributes to EDV and may be impaired (i.e., in atrial fibrillation). Static indices of the left side of the heart: • Left ventricular end-diastolic volume (LVEDV) • Left ventricular end-diastolic pressure (LVEDP) • Pulmonary artery occlusion or “wedge” pressure (PAOP or PAWP) Static indices of the right side of the heart: For the left side of the heart these relationships are as follows: For the right side of the heart these relationships are as follows: PAOP overestimates LVEDP in the following conditions: PAOP underestimates LVEDP in the following conditions: The PAOP does not predict preload and bears little or no relationship to the subsequent response to a volume challenge. It is important to realize, however, that pressure is one of the driving forces responsible for edema formation in the lungs. In this regard, the PAOP may function as more of a safety limit rather than as a guide to therapy.5–7 The CVP reflects RAP and has been traditionally used as a marker of right ventricular preload and, assuming a relationship between CVP, RVEDP, and RVEDV, of left ventricular preload. However, CVP does not accurately reflect left ventricular preload and has been shown to correlate poorly with blood volume.5–7 The main clinical value of measuring CVP is to provide information about the right side of the heart, as in cases of right ventricular failure or in assessing the right ventricular response to pulmonary hypertension. A low CVP in the setting of clinical signs of tissue hypoperfusion may be predictive of a benefit from fluid administration, and a rapid significant rise of CVP during a fluid challenge may indicate that the heart is not fluid responsive.8–12 Based on cardiac output measurements using thermodilution and incorporating indicator passage times, volumetric preload parameters have been introduced as an alternative to conventional “static” parameters and have become available with thermodilution-based monitoring techniques such as the PAC, pulse contour continuous cardiac output (PiCCO), and EV1000 systems. The continuous end-diastolic volume index (CEDVI), derived from a modified PAC is a surrogate of RVEDV. Global end-diastolic volume index (GEDVI), intrathoracic blood volume index (ITBVI), and extravascular lung water index (EVLWI) are parameters provided by devices that require transpulmonary thermodilution for the measurement of cardiac output based on pulse pressure analysis (i.e., PiCCO and EV1000 systems, see later). GEDVI represents the total intracardiac volume, and the volume of the descending aorta has been shown to be a better indicator of preload than CVP.13,14 Based on the study of heart-lung-interactions a number of so-called dynamic preload indicators have been introduced. Although these parameters are not measures of preload per se, they play an important role in the prediction of the hemodynamic response (i.e., by an increased cardiac output) to an increase in preload (preload or “volume” responsiveness). Generally, these parameters are based on the observation that transient changes in preload occur during mechanical ventilation.15,16 During mechanical ventilation each positive-pressure breath induces a decrease in venous return. In conditions of decreased volume status, when the right ventricle is responsive to an increase in preload, a reduction in right ventricular outflow will occur and result in a decrease of left ventricular preload after the number of cardiac cycles it takes to pump blood through the lung (pulmonary transit time). If the left ventricle is preload responsive as well, a transient decrease in stroke volume will occur. This cyclic variation in stroke volume (stroke volume variation, SVV) can indicate volume responsiveness if it reaches a certain degree and may describe the response to a volume challenge in relation to changes in cardiac output.17–21 Similar to SSV, a marked respiratory variation of the arterial pulse pressure (pulse pressure variation, PPV) has been found to accurately predict volume responsiveness.22–24 The pulse oximeter plethysmographic waveform has been shown to display respiratory variations during mechanical ventilation, which can be predictive of volume responsiveness. Calculation of the plethysmographic variability index (PVI) is performed by measuring changes of the perfusion index (PI = pulsatile infrared signal/nonpulsatile infrared signal × 100) over at least one respiratory cycle.25–27 Finally, respiratory variations of the inferior vena cava diameter as measured by transthoracic echocardiography (TTE) or the superior vena cava (SVC) diameter as measured by transesophageal echocardiography (TEE) have been shown to accurately predict volume responsiveness.28,29 Factors that increase myocardial contractility: • Drugs (inotropes, digoxin, calcium solutions) • Metabolic state (i.e., hyperthermia) • Sympathetic nervous system activity The following factors reduce myocardial contractility: • Metabolic state (acidosis, hypothermia, hyperkalemia, hypocalcemia) • Drugs (e.g., β-adrenergic receptor antagonists, digoxin) • Acute and chronic cardiomyopathies where T = tension, P = intraventricular pressure, and r = intraventricular radius. In this equation afterload is mainly dependent on wall thickness and ventricular radius. At a given pressure, an increase in ventricular radius (i.e., dilatation of the ventricle) will increase afterload. An increase in wall thickness (i.e., ventricular hypertrophy) will reduce afterload. Afterload for the left ventricle is increased by the following factors: • Anatomic obstruction (e.g., aortic valve stenosis, subvalvular obstruction) • Raised systemic vascular resistance (SVR) • Decreased elasticity of the aorta and great vessels Afterload for the right ventricle is increased by the following factors: • Anatomic obstruction (e.g., pulmonary valve stenosis) • Raised pulmonary vascular resistance (PVR) (e.g., pulmonary hypertension, pulmonary embolism, hypoxia, and hypercarbia) • Flow component—frictional opposition to blood flow through the vessels, with blood viscosity being the major determinant: Vascular resistance increases with an increase in viscosity. • Frequency-dependent or “reactive” component—related to the compliance of the vessel walls and the inertia of the ejected blood. A low vascular compliance increases vascular resistance. SVR and PVR cannot be measured directly and thus are calculated from Ohm’s law: where V = voltage, I = current, and R = resistance. Applied to cardiovascular physiology this principle looks as follows: in which ΔP is the pressure gradient, CO (cardiac output) is “flow,” and R is the resistance. where MAP = mean arterial pressure (mm Hg), RAP = right atrial pressure (mm Hg), and CO = cardiac output. To convert from mm Hg to dyne × second/cm5 the multiplication by 80 is necessary. Normal values for SVR range from 800 to 1200 dyne × second/cm5. If standardized to body surface area, SVR is quoted as SVRI (SVR index) with normal values for SVRI ranging from 1900 to 2400 dyne × second/cm5/m2. The following factors affect SVR: • Vessel diameter: According to the law of Hagen-Poiseuille resistance is inversely related to the fourth power of the radius. This means that a decrease in vessel diameter during vasoconstriction will result in a significant increase in SVR. Vasodilation decreases SVR. • Compliance of the systemic circulation Values for PVR are normally below 250 dyne × second/cm5. Again, PVR is often quoted as the PVR index or PVRI with a normal range between 255 to 285 dyne × second/cm5/m2. The following factors affect PVR: Although the concept of vascular resistance permits an intuitive understanding of the circulation, a number of limitations make it unreasonable to use SVR and PVR to guide therapy. First, because SVR and PVR are derived variables, errors inherent in the measurement of their components (i.e., filling pressures) are multiplied, making the derived number less accurate. Further, these calculations assume a linear relation between pressure and flow, which does not exist in vivo (Fig. 3.2). It is therefore wise to use these parameters with caution and consider their limitations in clinical practice. It has already been stressed that accurate assessment of the preload status of the heart is important because it may allow for the application of interventions resulting in an improvement of cardiovascular dynamics. However, without knowing whether the heart will actually respond to an increase of a certain status of volume or preload, this information is only of limited value. This concept—the individual response of cardiac output to a volume challenge—has become known as volume responsiveness.30 Intravascular volume expansion is often the initial intervention in patients with circulatory failure. However, only approximately 50% of patients given a fluid challenge will respond by increasing cardiac output. The ability to distinguish fluid-responsive patients from nonresponders is important to avoid inappropriate and potentially harmful fluid challenges in situations in which other interventions (i.e., inotropes or vasopressors) should be preferentially used. Consequences of inappropriate fluid therapy include pulmonary edema, deterioration in gas exchange, and cardiac failure. Conventional static markers of cardiac preload, such as CVP and PAOP, are poor predictors of fluid responsiveness. CVP and PAOP reflect intracavitary pressures that frequently show poor correlation with transmural pressures, which are actually related to EDV through chamber compliance. Therefore, EDV is overestimated (i.e., under the application of PEEP) or underestimated (i.e., in concentric left ventricular hypertrophy) in many clinical situations. Furthermore, static parameters do not determine the position of the heart on the “individual” Frank-Starling curve as contractile function is not taken into the account. A patient may fail to respond to a fluid challenge because of ventricular dysfunction or poor ventricular compliance (see Fig. 3.1
Assessment of Cardiac Filling and Blood Flow
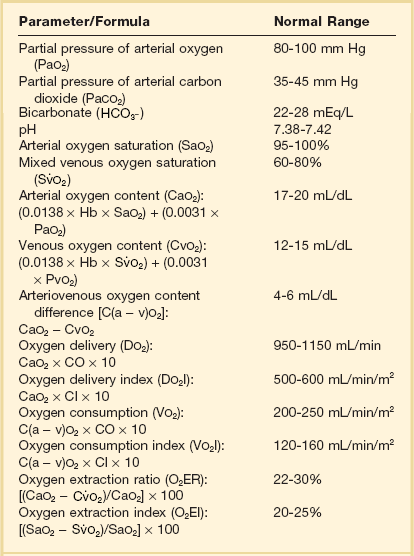
Parameter/Formula
Normal Range
Arterial blood pressure (BP)
Systolic (SBP)
90-140 mm Hg
Diastolic (DBP)
60-90 mm Hg
Mean arterial pressure (MAP):
[SBP + (2 × DBP)]/3
70-105 mm Hg
Right atrial pressure (RAP)
2-6 mm Hg
Right ventricular pressure (RVP)
Systolic (RVSP)
15-25 mm Hg
Diastolic (RVDP)
0-8 mm Hg
Pulmonary artery pressure (PAP)
Systolic (PASP)
15-25 mm Hg
Diastolic (PADP)
8-15 mm Hg
Mean pulmonary artery pressure (MPAP):
[PASP + (2 × PADP)]/3
10-20 mm Hg
Pulmonary artery occlusion pressure (PAOP)
6-12 mm Hg
Left atrial pressure (LAP)
6-12 mm Hg
Cardiac output (CO):
HR × SV/1000
4.0-8.0 L/min
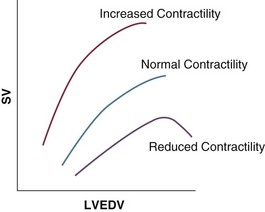
Derived Parameter/Formula
Normal Range
Cardiac index (CI):
CO/BSA
2.5-4.0 L/min/m2
Stroke volume (SV):
CO/HR × 1000
60-100 mL/beat
Stroke volume index (SVI):
CI/HR × 1000
33-47 mL/m2/beat
Systemic vascular resistance (SVR):
80 × (MAP − RAP)/CO
1000-1500 dyn⋅s/cm5
Systemic vascular resistance index (SVRI):
80 × (MAP − RAP)/CI
1970-2390 dyn⋅s/cm5/m2
Pulmonary vascular resistance (PVR):
80 × (MPAP − PAOP)/CO
<250 dyn⋅s/cm5
Pulmonary vascular resistance index (PVRI):
80 × (MPAP − PAOP)/CI
255-285 dyn⋅s/cm5/m2
Cardiac Filling
Preload
Static Indicators of Preload
Volumetric Indicators of Preload
Dynamic Indicators of Preload (or Preload Responsiveness)
Stroke Volume Variation
Pulse Pressure Variation
Plethysmographic Variability Index
Indices Derived by Echocardiography
Contractility
Afterload
Indicators of Afterload
Clinical Limitations of Systemic Vascular Resistance and Pulmonary Vascular Resistance
Clinical Interpretation of Indicators of Preload
Static Indicators of Preload

Full access? Get Clinical Tree


Assessment of Cardiac Filling and Blood Flow