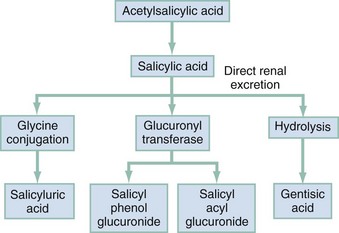
Chapter 149 Unfortunately, the severity of this poisoning may be underestimated because of the lack of familiarity with the clinical picture. Salicylate toxicity can cause metabolic acidosis, seizure, hyperthermia, pulmonary edema, cerebral edema, renal failure, and death. Morbidity and mortality are increased by delayed diagnosis in elderly patients with chronic medical problems and in young patients diagnosed with an acute illness.1 Salts of salicylic acid are rapidly absorbed intact from the gastrointestinal tract, with appreciable serum concentrations occurring within 30 minutes. Two thirds of a therapeutic dose is absorbed in 1 hour, and peak levels occur in 2 to 4 hours. Large ingestions frequently delay gastric emptying, and ingestions of enteric capsules may cause a prolonged absorption with rising serum levels for 12 hours or more.2 In the intestinal wall, liver, and red blood cells, aspirin is hydrolyzed to free salicylic acid, which reversibly binds to albumin (which contains a genetically determined variable number of salicylate-binding sites). In the liver, salicylate is conjugated with glucuronic acid and glycine (Fig. 149-1). A small fraction is hydroxylated. Free salicylate and its conjugates are eliminated by renal excretion. At therapeutic salicylate concentrations, elimination follows first-order kinetics, and excretion is proportional to salicylate concentration. When serum salicylate concentrations are greater than 30 mg/dL, however, elimination follows zero-order kinetics, and the metabolic rate is constant. The metabolic pathways become saturated, and the pH-sensitive urinary excretion of salicylic acid determines the half-life, which becomes prolonged and may approach 15 to 30 hours with toxic doses.3 Acid-Base Disturbances and Metabolic Effects.: Salicylate stimulates the medullary respiratory center early and increases the sensitivity of the respiratory center to pH and carbon dioxide partial pressure (PCO2). Hyperventilation develops early, then subsequently becomes a compensatory mechanism to the metabolic acidosis. Prolonged high serum concentrations eventually depress the respiratory center. Respiratory alkalosis is compensated by the buffering of the hemoglobin-oxyhemoglobin system, the exchange of intracellular hydrogen ions for extracellular cations, and the urinary excretion of bicarbonate. Loss of bicarbonate decreases buffering capacity and intensifies the metabolic acidosis.4,5 Toxicity results primarily from salicylate interference with aerobic metabolism by uncoupling of mitochondrial oxidative phosphorylation. Inhibition of the Krebs dehydrogenase cycle increases production of pyruvic acid and increases conversion to lactic acid. Increased lipid metabolism increases production of ketone bodies. Metabolic rate, temperature, tissue carbon dioxide, and oxygen consumption are increased. Tissue glycolysis predisposes to hypoglycemia. (Hepatic gluconeogenesis and release of epinephrine may cause the less common hyperglycemia.) Inefficiency of anaerobic metabolism results in less energy being used to create adenosine triphosphate, and energy is released as heat, causing the hyperthermia frequently seen in salicylate poisoning.4 Only nonionized particles can cross the lipophilic cell membrane and accumulate in the brain and other tissues. Because ASA has a low pKa,3,5 the majority of salicylate is ionized, and little salicylate enters tissues at the physiologic pH of 7.4. However, as pH decreases, more particles become un-ionized and cross the cell membrane and blood-brain barrier, markedly increasing the movement of salicylate into the tissues and central nervous system (CNS).4,5 Fluid and Electrolyte Abnormalities.: Significant potassium loss in salicylate toxicity is caused by vomiting, secondary to stimulation of the medullary chemoreceptor trigger zone; increased renal excretion of sodium, bicarbonate, and potassium as a compensatory response to the respiratory alkalosis; salicylate-induced increased permeability of the renal tubules with further loss of potassium; intracellular accumulation of sodium and water; and inhibition of the active transport system, secondary to uncoupling of oxidative phosphorylation. The net result is rapid depletion of potassium stores.4 A salicylate-induced decrease in renal blood flow or direct nephrotoxicity may cause acute nonoliguric renal failure. Salicylate-induced secretion of inappropriate antidiuretic hormone may also affect renal function.6 Pulmonary and Cerebral Edema.: The exact mechanism by which salicylates increase alveolar capillary membrane permeability is unknown. Theories include inhibition of prostacyclin, changes in platelet-vessel interaction, and neurogenic influences. In adults, the risk factors for salicylate-induced pulmonary edema include age older than 30 years, cigarette smoking, chronic salicylate ingestion, metabolic acidosis, neurologic symptoms, and serum salicylate concentration greater than 40 mg/dL. Risk factors in children include high serum salicylate levels, large anion gap, decreased serum potassium concentration, and low PCO2.7 Chronic Ingestion Physiology.: Physiologic changes of aging predispose elderly patients to toxicity from chronic therapeutic ingestion. Decreased liver blood flow rates decrease biotransformation of salicylate, and decreased renal function decreases salicylate clearance. Chronic ingestion allows salicylates more time to pass through the blood-brain barrier. In addition, chronic ingestion of aspirin decreases albumin binding, increasing the free salicylate that can enter the cell. This causes significant clinical illness with a relatively low serum salicylate concentration. A patient with chronic salicylate toxicity and a serum concentration of 40 mg/dL may be more ill than a patient with an acute ingestion and serum concentration of 80 mg/dL.8 Shortness of breath and altered sensorium are caused by pulmonary and cerebral edema, respectively. Noncardiac pulmonary edema may be more common in children than scattered case reports suggest.9 Failure to recognize salicylate toxicity as the cause of pulmonary edema increases the likelihood of morbidity and mortality in these patients. Low pH and bicarbonate level portend severe disease. The pH begins to drop when the patient is unable to compensate for the acidosis. Lactic acid accumulates, and serum bicarbonate is consumed. When pH is less than 7.4 and both PCO2 and bicarbonate level are low, the patient begins to decompensate hemodynamically. In the intubated patient or the acidotic patient with low PCO2 and bicarbonate level, hemodialysis should be undertaken.10 The symptoms of salicylism (hyperthermia, altered mental status or coma, pulmonary edema, and shock) mimic sepsis and the symptoms of many other diseases (Box 149-1). This is especially true with chronic ingestion; serum salicylate concentration is relatively low, and the severity of the poisoning is not recognized.11 Death is caused by CNS depression and cardiovascular collapse. Specific treatment of salicylate toxicity has two main objectives: (1) to correct fluid deficits and acid-base abnormalities and (2) to increase excretion (Box 149-2). Electrolyte values are helpful to guide replacement and to assess renal function necessary to excrete salicylates. Serum salicylate levels should be repeated until they are decreasing to measure the effectiveness of treatment and to guide the decision for dialysis.
Aspirin and Nonsteroidal Agents
Aspirin
Principles of Disease
Pathophysiology
Clinical Features
Diagnostic Strategies
Differential Considerations
Management
Aspirin and Nonsteroidal Agents