175 Antidepressant Drug Overdose
Major depressive disorder (MDD) is a common and extremely important disease. The most recent national survey found a prevalence of 6.6% during the preceding 12 months and estimated that 16.2% of Americans will experience MDD during their lifetime.1 The treatment of MDD underwent a major revolution in the 1950s and 1960s with the introduction of the tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs). Subsequently, the development of the so-called selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs) allowed effective treatment of depression without most of the side effects and toxicity associated with the older classes of medications. This favorable side-effect profile has led to a fundamental shift away from the use of TCAs and MAOIs and has dramatically increased the number of patients taking antidepressant medications.
Since they are used to treat MDD, it is not surprising that antidepressants have always figured prominently on the list of drugs used during intentional self-poisonings. According to data published by the American Association of Poison Control Centers, antidepressants have been the third most commonly ingested class of medications, after analgesics and sedatives/hypnotics/antipsychotics, for the past 15 years.2–4 As the use of TCAs and MAOIs has declined, so have the number of fatalities associated with these overdoses. In 1998, antidepressants were associated with almost 20% of fatal drug ingestions, but by 2008, this number had dropped to 8%.2,3 Despite this dramatic decrease, antidepressants remain the third most common cause of fatal drug ingestions.2
 Classification
Classification
As shown in Table 175-1, the most commonly used classification scheme divides antidepressant medications into tricyclic antidepressants, monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, and a miscellaneous group of drugs referred to as atypical antidepressants.5–7 This classification is suboptimal from a pharmacologic standpoint because it mixes structural (TCA) and functional (e.g., SSRI, MAOI) drug characteristics. In addition, as discussed later, functional characteristics can vary markedly among the drugs in each category, and significant overlap can occur between categories. Nevertheless, this classification scheme does provide a framework for discussing the pharmacology, clinical manifestations, and management of antidepressant overdose.
TABLE 175-1 Classification of Antidepressant Medications
| Generic Name | Brand Name |
|---|---|
| Tricyclic Antidepressants | |
| Amitriptyline | Elavil |
| Amoxapine | Asendin |
| Clomipramine | Anafranil |
| Desipramine | Norpramin |
| Doxepin | Adapin, Sinequan |
| Imipramine | Tofranil |
| Maprotiline | Ludiomil |
| Nortriptyline | Pamelor |
| Protriptyline | Vivactil |
| Trimipramine | Surmontil |
| Monoamine Oxidase Inhibitors | |
| Isocarboxazid | Marplan |
| Phenelzine | Nardil |
| Tranylcypromine | Parnate |
| Moclobemide | Manerix |
| Selective Serotonin Reuptake Inhibitors | |
| Citalopram | Celexa |
| Escitalopram | Lexapro |
| Fluoxetine | Prozac |
| Fluvoxamine | Luvox |
| Paroxetine | Paxil |
| Sertraline | Zoloft |
| Serotonin and Norepinephrine Reuptake Inhibitors | |
| Venlafaxine | Effexor |
| Desvenlafaxine | Pristiq |
| Duloxetine | Cymbalta |
| Milnacipran | Savella |
| Atypical Antidepressants | |
| Bupropion | Wellbutrin |
| Mirtazapine | Remeron |
| Reboxetine | Edronax |
| Nefazodone | Serzone |
| Trazodone | Desyrel |
 Pharmacology
Pharmacology
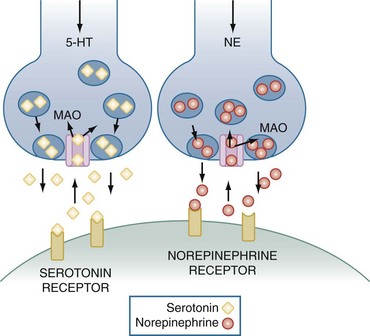
Before describing the pharmacology of the antidepressant drugs, it is important to review the release, reuptake, and metabolism of serotonin (5-hydroxytryptamine [5-HT]) and norepinephrine (NE), two monoamine neurotransmitters that are believed to play a major role in the pathogenesis of depression. As illustrated in Figure 175-1, 5-HT and NE are each synthesized by specific neurons and packaged into vesicles in the presynaptic nerve terminal. An action potential causes these vesicles to fuse with the nerve membrane, thereby releasing 5-HT or NE into the synaptic cleft. After release, these neurotransmitters bind to specific postsynaptic receptors. Seven serotonin receptor families (designated 5-HT1, 5-HT2, and so forth) have been identified, and many contain more than one receptor subtype (e.g., 5-HT1A, 5-HT1B).8 Each family and each receptor subtype appears to have specific functions and distributions throughout the body, although all are present in the central nervous system (CNS). NE binds to two major families of postsynaptic receptors termed α and β, and each has two major subtypes, referred to as α1, α2, β1, and β2. After release, the actions of 5-HT and NE are terminated primarily by active reuptake into the presynaptic neuron by amine-specific transporters. There, they are either repackaged into vesicles for future release or inactivated by the mitochondrial-bound enzyme, monoamine oxidase (MAO). MAO has the important role of inactivating a wide variety of monoamines and is found in the brain, gastrointestinal (GI) tract, and liver as well as other organs and tissues. There are two enzyme subtypes. MAO-A primarily functions to inactivate 5-HT, NE, and tyramine, whereas dopamine, phenylethylamine, tyramine, and tryptamine are the major substrates of MAO-B.8
Pharmacologic Actions
Most antidepressant medications act to increase the extraneuronal concentrations of serotonin and/or norepinephrine in the CNS. The TCAs, SSRIs, and SNRIs do this by inactivating specific transporters in the presynaptic neuron, thereby preventing the reuptake of these biogenic amines from the synaptic cleft. As shown in Table 175-2, the TCAs have a wide range of potencies and specificities for the 5-HT and NE transporters.5,9 For example, desipramine is the most potent inhibitor of NE reuptake, whereas clomipramine is the most effective serotonin reuptake blocker. The SSRIs, although much more specific, also demonstrate variable potency for transporter blockade.5,10–13 The SNRIs inhibit both 5-HT and NE reuptake, but with the exception of duloxetine have relatively low potency.5,14,15 At present, it is not clear that differences in drug selectivity translate into differences in efficacy, and differences in potency are largely eliminated through dosage adjustments.
TABLE 175-2 Potencies of Antidepressants for Blocking Neurotransmitter Reuptake
| Drug | Norepinephrine (NE) | Serotonin (5-HT) |
|---|---|---|
| Tricyclic Antidepressants | ||
| Desipramine | +++++ | ++ |
| Protriptyline | ++++ | ++ |
| Nortriptyline | +++ | + |
| Amoxapine | ++ | + |
| Doxepin | ++ | + |
| Clomipramine | + | +++++ |
| Imipramine | + | ++++ |
| Amitriptyline | + | ++ |
| Selective Serotonin Reuptake Inhibitors | ||
| Paroxetine | + | +++++ |
| Sertraline | ± | ++++ |
| Escitalopram | — | ++++ |
| Citalopram | — | +++ |
| Fluoxetine | ± | +++ |
| Fluvoxamine | — | ++ |
| Serotonin and Norepinephrine Reuptake Inhibitors | ||
| Duloxetine | +++ | +++++ |
| Venlafaxine | ± | + |
| Desvenlafaxine | + | ++ |
| Milnacipran | + | + |
Potency increases progressively from ± to +++++. —, no effect.
MAOIs prevent the breakdown of 5-HT and NE after reuptake has occurred.16 The antidepressant effect of these drugs requires the inhibition of MAO-A and is presumed to result from increased concentrations of 5-HT and NE in the brain. Most MAOIs, including isocarboxazid, phenelzine, and tranylcypromine, irreversibly inactivate both MAO-A and MAO-B. Recently, several new drugs which selectively and reversibly inactivate MAO-A have been developed. The most widely studied of these drugs, moclobemide, has been approved for use in several European countries but is not yet available in the United States.
The so-called atypical antidepressants act through a variety of different mechanisms.6,7,17 Bupropion primarily inhibits the reuptake of dopamine by blocking specific presynaptic transporters. Mirtazapine is a potent central α-adrenergic agonist that promotes the release of both serotonin and norepinephrine. It also acts as an antagonist at 5-HT2 and 5-HT3 receptors. Reboxetine is a selective inhibitor of NE reuptake. Nefazodone and trazodone act primarily by blocking 5-HT2A receptors.
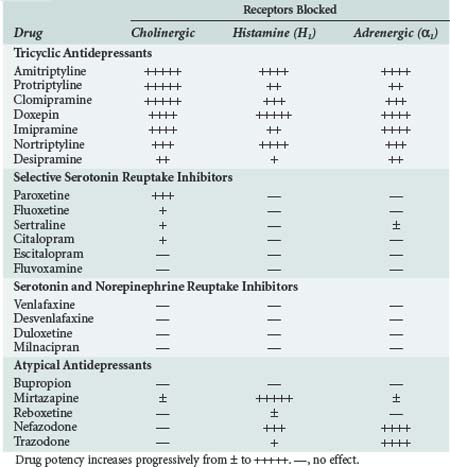
In addition to their therapeutic effects, many of the antidepressant drugs also have a variety of undesirable properties. As shown in Table 175-3, many of them block α1-adrenergic, cholinergic, and/or histamine (H1) receptors.5–15 The TCAs are the most potent antagonists of all three receptor types, although the atypical antidepressants, mirtazapine, nefazodone, and trazodone, also block H1 and/or α1-adrenergic receptors. In general, the SSRIs and SNRIs have little or no effect on these receptors. The TCAs also block fast inward sodium channels on myocardial cells, which is analogous to the effect of type I antiarrhythmic drugs.
Absorption, Distribution, Metabolism, and Excretion
In general, all antidepressants are well absorbed after oral administration, and peak plasma concentrations are usually achieved within several hours. Once absorbed, the TCAs in particular become tightly bound to plasma proteins and have a large volume of distribution. The MAOIs are metabolized primarily by hepatic acetylation, and the rate at which this process occurs varies widely among the population. Inactivation of the TCAs, SSRIs, SNRIs, and atypical antidepressants occurs largely via hepatic CYP450 enzymes, and the final byproduct is excreted in the urine. This means that coadministration of these drugs or use of another medication that inhibits CYP450 function may lead to significant drug toxicity.5,9,18
The duration of action of the antidepressants depends on the clearance rate of the parent compound as well as that of any active metabolites. Except for moclobemide, which is reversible and short acting, irreversible enzyme inactivation by the MAOIs causes their effects to last up to 2 weeks after these drugs have been ingested. In general, the other antidepressant drugs have half-lives in the range of 20 to 40 hours.5 Exceptions are fluoxetine, and its active metabolite norfluoxetine, which have half-lives of about 2 and 10 days, respectively; and venlafaxine and nefazodone, which have half-lives of approximately 5 and 3 hours, respectively.5 Because it takes approximately five half-lives for complete drug elimination to occur, most of the antidepressants can have prolonged effects after a toxic ingestion.
 Toxicology
Toxicology
The symptoms and signs that accompany an overdose, the severity and duration of toxicity, and even specific therapeutic strategies can be predicted based on a knowledge of the pharmacologic actions of each of the antidepressant drugs. Because of their potent antagonistic effects at cholinergic, adrenergic, and histaminic receptors and their ability to block sodium channels in the myocardium, TCAs are the most likely class of antidepressant drugs to cause major morbidity or death when taken in overdose.19 Not surprisingly, significant morbidity and mortality are very uncommon following ingestion of the SSRIs and SNRIs, which lack these properties.19 MAOIs and the atypical antidepressants have an intermediate toxicity profile.
Tricyclic Antidepressants
Clinical Features
The manifestations of TCA overdose are caused by the receptor and sodium channel blocking properties of these drugs.19,20 Patients typically present with symptoms and signs of an anticholinergic syndrome, or toxidrome, which may include mydriasis, dry mouth, slowed intestinal peristalsis or ileus, urinary retention, fever, flushing, sinus tachycardia, CNS depression that ranges from lethargy to coma, respiratory depression, and seizures. Blockade of α1-adrenergic receptors causes vasodilation, which decreases preload and vascular resistance and can lead to hypotension. Through their direct toxic effect on the myocardium, TCA overdose may slow depolarization and lead to prolongation of the QRS and QT intervals, heart block, and ventricular arrhythmias. Inhibition of the sodium current may also lead to decreases in myocardial contractility, stroke volume, and cardiac output. Hypotension can result from vasodilation, impaired contractility, or both. The life-threatening complications of TCA overdose, therefore, are ventricular arrhythmias, advanced heart block, shock, stupor and coma, respiratory depression, and recurrent generalized seizures.

Full access? Get Clinical Tree