What is cachexia?
Cachexia, anorexia, and fatigue are an overlapping and often neglected group of symptoms that at some stage affect most patients with cancer. Similar symptoms may be seen in other conditions, including advanced cardiac failure, COPD, renal failure, and AIDS and in patients who have been in intensive care units. The term cachexia is derived from the Greek words kakos and hexis meaning poor condition. Cachexia is a broad heterogeneous syndrome. The key feature is wasting that cannot be easily or completely reversed by an increase in food intake alone. Anorexia or reduced appetite often accompanies cachexia. Some patients with anorexia, however, do not have cachexia. Equally some cachectic patients become wasted but apparently do not have anorexia. Fatigue is a common element but again this can occur in isolation.
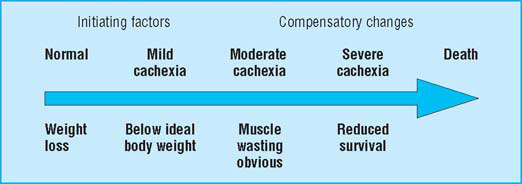
Cachexia is complex and multifactorial. A patient’s evident chronic negative energy and protein balance is most commonly driven by a combination of reduced food intake and metabolic change. Symptoms can include anorexia, early satiety, taste changes, loss of physical function, and fatigue. Signs may include muscle wasting, loss of subcutaneous fat, and peripheral oedema. Different symptoms may predominate in individual patients and may also change with time. Advanced cachexia is generally easy to recognise, but the early symptoms may be more subtle. An unintentional loss of weight of more than 10% with an appropriate underlying diagnosis has traditionally been used as a definition of cachexia. This definition, however, neglects other relevant symptoms and if used rigidly is likely to delay diagnosis and therefore treatment. Equally, with an ever increasing tendency towards obesity in the general population, lesser degrees of weight loss are likely to identify a proportion of patients who, while at risk of developing cachexia, may still be above ideal body weight.
Why is cachexia important?
Anorexia and fatigue are consistently among the most common symptoms reported by patients with advanced cancer. Cachexia affects over 80% of such patients or patients with AIDS before death. It is particularly common in those with solid tumours of the upper gastrointestinal tract and lung. Those with cachexia have reduced survival, often experience anorexia and fatigue, have an altered body image, and have impaired physical activity and overall quality of life. Response to antineoplastic therapy is reduced and morbidity caused by treatment increased. Cachexia is usually progressive and is sometimes fatal.
Does this patient have cachexia?
A history and physical examination are probably the most useful tools in making the diagnosis and assessing response to therapy. Weight loss in the past six months should be recorded. Symptoms associated with reduced food intake (for example, loss of appetite, early satiety, nausea or vomiting, and alterations in taste or smell) are key warning signals. Weight should be measured and recorded along with height. Oedema and ascites are common and should be documented because fluid retention may mask the severity of underlying weight loss. Body mass index (weight (kg)/height (m)2) should be calculated. A BMI <18 indicates severe undernutrition. The plasma albumin concentration may be low and, if it is accompanied by a raised C reactive protein (CRP) or erythrocyte sedimentation rate (ESR), probably reflects an underlying systemic inflammatory response that may contribute to the weight loss.
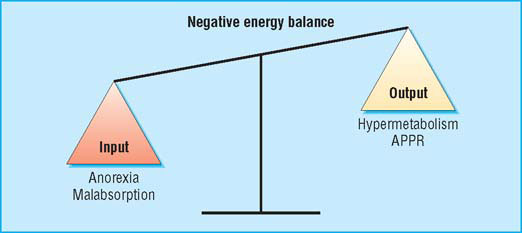
Cachexia is multifactorial, and it effects a patient’s balance of negative energy (APPR = acute phase protein response)
The progression through cachexia
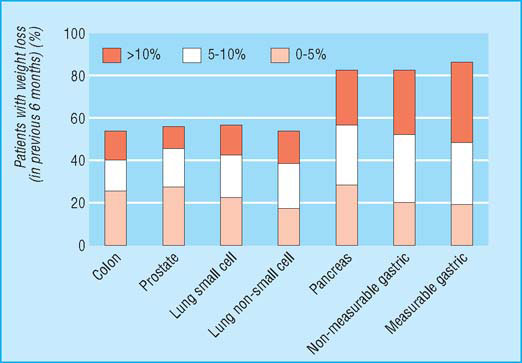
- 5% weight loss
- >15% weight loss (BMI 18; albumin <30 g/l)
Nutritional status of patients with cancer: prevalence and severity of weight loss
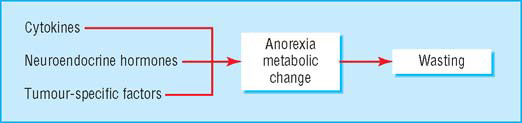
Why do patients become cachectic?
The cachectic patient is like an accelerating car running out of petrol. Anorexia critically reduces fuel supply (by about 300–500 kcal (1254–2090 kJ) a day), while accelerated metabolic cycling (for example, glucose-lactate cycling) drives hypermetabolism (100–200 kcal a day). In addition, there are direct catabolic effects at the level of skeletal muscle (for example, activation of the ubiquitin-proteasome pathway) and adipose tissue. The mediators of these changes are complex and include proinflammatory cytokines, stress hormones, and tumour specific cachectic factors such as proteolysis inducing factor (PIF). The main energy (subcutaneous fat) and labile protein reserves (skeletal muscle) of the body are mobilised and the patient becomes prone to secondary effects such as insulin resistance and further muscle wasting due to immobility. These changes underlie a key paradox of cachexia in that while the metabolic rate may be increased, overall (or total) energy expenditure is decreased due to a fall in physical activity.
Management
Once a patient has become severely wasted and bed bound and is within weeks of dying it is unlikely that intervention can have any objective benefit. The longstanding practice of giving such patients steroids to improve mood and perhaps appetite remains a keystone of clinical practice.
At the other extreme of intervention is the patient with incurable cancer, who has a prognosis measured in months, but who has malnutrition related to gut failure because of localised and relatively stable intra-abdominal malignancy. A proportion of these patients benefit from total parenteral nutrition at home. Although not often used in the UK, such intervention, if guided by expert clinical judgment, can result in improved quality and quantity of life. Other groups who may benefit from artificial nutritional support but via the enteral route include patients with slowly advancing head and neck tumours.
Therapeutic principles of management
For most patients the management of cachexia requires insight and enthusiasm from the physician, surgeon, general practitioner, nurse specialist, and dietician with whom the patient may come into contact. Cachexia is a chronic problem for which there is no quick fix and which requires repeated re-evaluation as the clinical condition of the patient changes. Once signs of cachexia are evident patients generally have two to six months to live. Early recognition and prophylactic measures are better than trying to reverse an advanced situation. Good clinical judgment is paramount to identify all reversible factors that may be contributing to the patient’s wasting syndrome. In particular, if nausea and vomiting can be controlled with regular antiemetics (or surgery if there is a defined mechanical obstruction), malabsorption treated with enzyme supplements, constipation treated with laxatives, pain well controlled with the minimum of sedation, and depression treated with antidepressants then this sets the background for optimal appetite and function of the gastrointestinal tract.
Food intake
With the recognition that weight loss in patients with cancer is most commonly due to a combination of reduced food intake and metabolic change, once the overall management of the cachectic patient has been optimised, therapeutic strategies should try to address both these issues.
Mediators of cachexia

Full access? Get Clinical Tree








