KEY POINTS
1. Despite the many and varied etiologies, the manifestations of pericardial disease are primarily expressed as pericardial effusion, inflammation, and constriction. The common theme is impaired cardiac filling and diastolic dysfunction.
2. A relatively small amount of fluid (50 to 100 mL) that accumulates acutely within the closed pericardial space is sufficient to dramatically increase intrapericardial pressure and interfere with cardiac filling. Conversely, a chronic increase in pericardial fluid will produce tamponade only after a large volume of fluid accumulation, perhaps as great as a liter.
3. The primary abnormality in cardiac tamponade is impaired cardiac filling caused by increased intrapericardial pressure. The right heart is most vulnerable to compression due to its thin walls and lower chamber pressures.
4. Tamponade is often described as Beck’s Triad: muffled heart sounds, jugular venous distension, and hypotension.
5. Pulsus Paradoxus (drop in systolic pressure during inspiration) occurs secondary to septal shift crowding the left ventricle during right ventricular filling. The opposite occurs during expiration. This phenomenon is described as enhanced ventricular interdependence.
6. Electrical alternans is due to the swinging motion of the heart in the pericardial sac.
7. Diastolic collapse lasting more than one-third of diastole demonstrated on echocardiography is fairly specific for tamponade.
8. Anesthetic induction in patients with tamponade can lead to cardiovascular collapse. If pericardiocentesis cannot be performed in a compromised patient and a surgical procedure is utilized instead, the patient should be prepped and draped prior to anesthetic induction so that surgery can proceed immediately after intubation. Volume loading prior to general anesthetic induction as well as inotropes may be required. Expect further deterioration after positive pressure ventilation is initiated.
9. Constrictive pericarditis (CP) is a diagnosis that encompasses a wide-spectrum of disease, from acute and subacute cases that may resolve spontaneously or with medical therapy to classic chronic progressive CP.
10. Although manifestations of hemodynamic instability are less with chronic pericarditis than with tamponade, induction and maintenance of anesthesia must encompass the same concerns.

1
I. Introduction.
The clinical significance of pericardial disease encompasses a wide spectrum, ranging from asymptomatic subclinical disease to acute life-threatening emergencies. This spectrum is best appreciated with a thorough understanding of the physiologic derangements these disorders place upon the heart and its function. In addition, the perioperative care of these patients requires an appreciation of how this altered physiology is influenced by anesthetic techniques and pharmacology. Further complicating the management of these patients are the underlying conditions causing the pericardial dysfunction, which introduce additional management concerns of their own. Despite the many and varied etiologies, the manifestations of pericardial disease are primarily expressed as pericardial effusion, inflammation, and constriction. Regardless, the common theme of most all pericardial pathology is impaired cardiac filling and diastolic dysfunction. This chapter reviews the normal structure and function of the pericardium, as well as the most common etiologies leading to pericardial disease. The two most clinically relevant pericardial disorders will be discussed in detail: cardiac tamponade and constrictive pericarditis.
II. Pericardial anatomy and physiology
A. The normal pericardium is a dual-enveloped sac surrounding the heart and great vessels. It comprises two layers: the parietal pericardium and the visceral pericardium. The parietal pericardium is a thick, fibrous outer layer composed primarily of collagen and elastin. It attaches to the adventitia of the great vessels, diaphragm, sternum, and the vertebral bodies. The inner visceral pericardium rests on the surface of the heart. It is composed of a single layer of mesothelial cells, which adhere to the pericardium. Normal pericardial thickness is 1 to 2 mm.
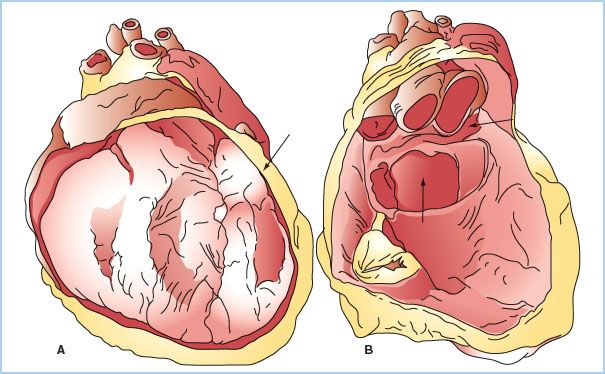
B. Two distinct sinuses are formed at points where the pericardium appears to fold onto itself. The oblique sinus forms posteriorly, between the left atrium and pulmonary veins, and is a common location for blood to collect after cardiac surgery. The transverse sinus also forms posteriorly behind the left atrium, situated behind the aorta and pulmonary artery (Fig. 20.1).
Figure 20.1 Anatomy of the pericardium and pericardial sinuses. The left image (A) demonstrates the heart in situ with a section of the parietal pericardium cut away. The left panel (B), with the heart cut away, demonstrates the oblique sinus (arrow at 6 o’clock) and the transverse sinus (arrow at 3 o’clock). (Reused from Lachman N, Syed FF, Habib A, et al. Correlative anatomy for the electrophysiologist, part 1: The pericardial space, oblique sinus, transverse sinus. J Cardiovasc Electrophysiol. 2010;21(suppl 12):1421–1426).
C. Normal cardiac function can still occur in the absence of the pericardium, making it nonessential for survival. However, it does provide several useful physiologic functions. It aids in the reducing friction between the heart and surrounding structures, limits acute dilatation of cardiac chambers, provides a barrier to infection, optimizes coupling of left and right ventricular filling and function, and limits excessive motion of the heart within the chest cavity. The pericardium is also metabolically active, secreting prostaglandins that affect coronary artery tone and cardiac reflexes [1].
D. The pericardium is a highly innervated structure. Pericardial inflammation or manipulation may produce severe pain or vagally mediated reflexes.
III. Causes of pericardial disease.
The etiologies of pericardial disease are numerous and can lead to pericardial inflammation, effusion, or both. Care of these patients must consider not only the pathological process of the pericardium, but the manifestations and complications of the underlying disease states. Pericardial disease can be caused by infection (e.g., viral, bacterial, fungal, tuberculosis), connective tissue disorders (e.g., systemic lupus erythematosus, sarcoidosis, rheumatoid arthritis), trauma, uremia, malignancy, post-myocardial infarction (Dressler’s Syndrome), or following cardiac surgery and other invasive cardiac procedures.
IV. Pericardial tamponade
A. Natural History
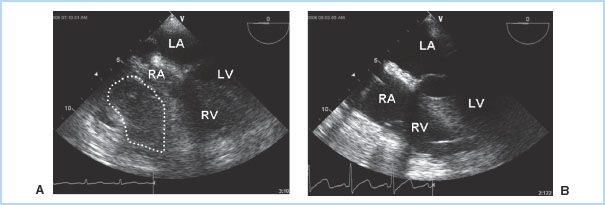
1. Etiology. The visceral pericardium is responsible for the production of pericardial fluid, which is an ultrafiltrate of plasma. This fluid provides lubrication to decrease friction between the pericardial layers. The pericardial space normally contains 10 to 50 mL of fluid, which is drained by the lymphatic system. As previously discussed, many conditions can cause fluid accumulation within the pericardial space, which may be serous, serosanguinous, purulent, or blood. However, the majority of cardiac effusions do not progress to tamponade. Tamponade occurs when the heart becomes extrinsically compressed by the contents of the pericardium, diminishing venous filling and, ultimately, cardiac output. In addition to effusion fluid, tamponade may also be caused by the accumulation of clot or air in the pericardial space. Acute, life-threatening tamponade most frequently results from bleeding into the pericardial space after cardiac surgery or other invasive cardiac procedures, following blunt chest trauma, or due to a ruptured ascending aortic aneurysm or aortic dissection [2]. Tamponade may occur in as many as 8.8% of patients presenting for cardiac surgery, although it is more commonly seen after valve surgery than coronary artery bypass grafting (CABG). The onset is often in the immediate postoperative period, but may occur as late as several days following surgery. Frequently there is localized clot or effusion, which causes nonuniform compression of the cardiac chambers and manifests without the classical clinical features of tamponade (Fig. 20.2). The diagnosis of post-cardiac surgery tamponade can therefore be challenging, especially when considering the many potential causes of hemodynamic instability during this time period. Unfortunately, morbidity and mortality increases significantly the longer the diagnosis is delayed [3].
Figure 20.2 Regional tamponade following cardiac surgery. In this transesophageal (TEE) midesophageal four- chamber view, a localized clot is seen compressing both the right atrium and right ventricle (A). Chamber compression is relieved following clot removal (B). RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle. (Fontes ML, Skubas N, Osorio J. Cardiac tamponade. In: Yao FF, Fontes ML, Malhotra V, eds. Yao & Artusio’s Anesthesiology: Problem Oriented Patient Management. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008:327.)
2
2. Symptomatology. Symptoms of cardiac tamponade are usually rapid in onset, but depend upon the rate at which pericardial fluid accumulates. A relatively small amount of fluid (50 to 100 mL) that accumulates acutely within the closed pericardial space is sufficient to dramatically increase intrapericardial pressure and interfere with cardiac filling. However, a chronic increase in pericardial fluid will produce tamponade only after a large volume is present (>1 L). A gradual accumulation of fluid stretches the parietal pericardium, allowing larger volumes to be tolerated before symptoms occur. Lack of this pericardial stretch explains the abrupt onset of symptoms and clinical deterioration in the setting of acute tamponade. The primary symptoms of cardiac tamponade include dyspnea, orthopnea, diaphoresis, and chest pain. Dyspnea is often the first and most sensitive symptom [4].
3
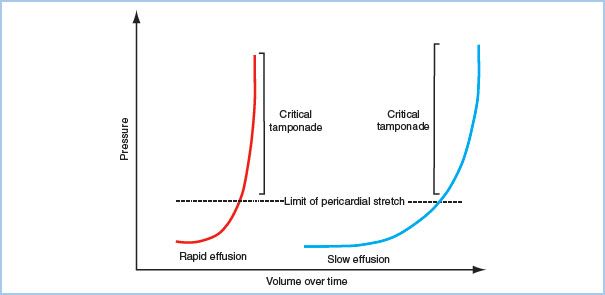
B. Pathophysiology. The primary abnormality in cardiac tamponade is impaired diastolic filling of the heart, caused by increased intrapericardial pressure that leads to compression of the atria and ventricles. The right heart is most vulnerable to the compression due to its thinner walls and lower chamber pressures. Diastolic filling pressures (e.g., central venous pressure [CVP], left atrial pressure [LAP], pulmonary capillary wedge pressure [PCWP], left ventricular end-diastolic pressure [LVEDP] and right ventricular end-diastolic pressure [RVEDP]) become elevated and are nearly equal to each other, as well as the intrapericardial pressure, hence the terminology in tamponade of equalization of pressures. Physiologic manifestations of pericardial fluid, as previously discussed, are contingent upon the rate and amount of fluid accumulation, with a continuum ranging from clinically insignificant to severe hemodynamic collapse (Fig. 20.3). Ventricular preload and volume is critically reduced, which translates into decreased stroke volume, cardiac output, and systemic blood pressure. Compensatory sympathetic responses attempt to offset this reduction in stroke volume, with elevated levels of plasma catecholamines resulting in systemic vasoconstriction and tachycardia. This may temporarily maintain cardiac output and systemic perfusion; however, sudden hemodynamic collapse may rapidly occur with the depletion of catecholamines and/or continued elevation of intrapericardial pressure.
Figure 20.3 Pressure–volume relationship in acute versus chronic pericardial effusions. Intrapericardial pressure is contingent upon the change in intrapericardial volume. Pressure is relatively stable until a critical volume occurs. At this point, minimal increases in volume will lead to significant changes in intrapericardial pressure. With chronic effusions, pericardial stretch allows for a greater amount of volume to accumulate before critical increases in pressure occur. The lack of pericardial stretch explains the significant elevations in pressures seen with only small amounts of rapidly accumulating intrapericardial fluid. (Figure from Avery EG, Shernan SK. Echocardiographic evaluation of pericardial disease. In: Savage RM, Aronson SA, Shernan SK, eds. Comprehensive Textbook of Perioperative Transesophageal Echocardiography. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2011:726.)
C. Diagnostic evaluation and assessment
4
1. Clinical evaluation
a. Acute tamponade is often described by Beck’s triad: muffled heart sounds, jugular venous distention (JVD) due to increased venous pressure, and hypotension. Other common findings include tachypnea and tachycardia.
5
b. Pulsus paradoxus although not specific for tamponade, may be observed. This is defined as a decrease of more than 10 mm Hg in systolic arterial pressure occurring with inspiration. Tamponade physiology leads to respiratory variability in ventricular diastolic filling where the negative intrathoracic pressure accompanying inspiration leads to enhanced right-sided filling. Since total intrapericardial volume is fixed by the effusion, as the right ventricle (RV) fills, it will lead to a shift of the interventricular septum toward the left ventricle (LV). This crowding of the LV impedes its filling, decreasing LV stroke volume and explaining the exaggerated decline in systolic blood pressure seen with inspiration. The opposite is true during expiration, with diminished RV and enhanced LV filling. This phenomenon is described as enhanced ventricular interdependence, in which the diastolic filling characteristics of one ventricle can have pronounced influence on the filling of the other ventricle. Pulsus paradoxus is also seen in patients with chronic lung disease, right ventricular dysfunction, and CP.
c. Chest x-rays may show an enlarged, globular, bottle-shaped cardiac silhouette, with widening of the mediastinum. The right costophrenic angle is reduced to less than 90 degrees and the lung fields are clear. Pericardial fat lines in a lateral film are an uncommon, but highly specific, finding.
6
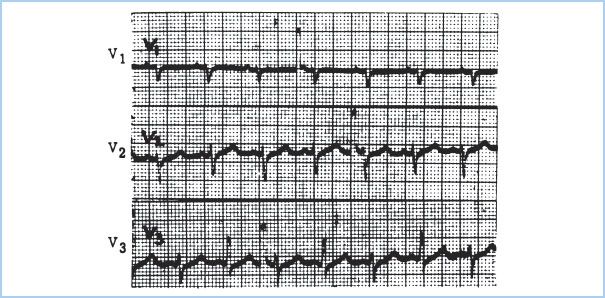
d. The ECG is nonspecific but may demonstrate sinus tachycardia, low-voltage QRS, nonspecific ST-T wave abnormalities, and electrical alternans. Electrical alternans (Fig. 20.4) is due to a swinging motion of the heart in the pericardial sac, leading to beat-to-beat changes in the electrical axis.
Figure 20.4 Electrical alternans with cardiac tamponade. Lead V3 demonstrates the variation in the R-wave axis in alternate beats. Note that this phenomenon is not seen in all electrocardiographic leads. (Figure from Hensley FA, Martin DE, Gravlee GR. A Practical Approach to Cardiac Anesthesia. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:476.)

Full access? Get Clinical Tree








