CHAPTER 28 Anesthesia for Organ Transplantation
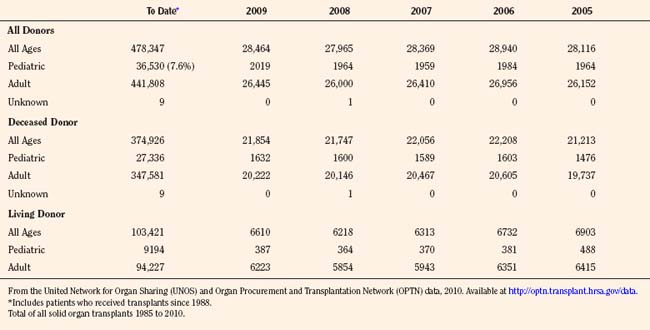
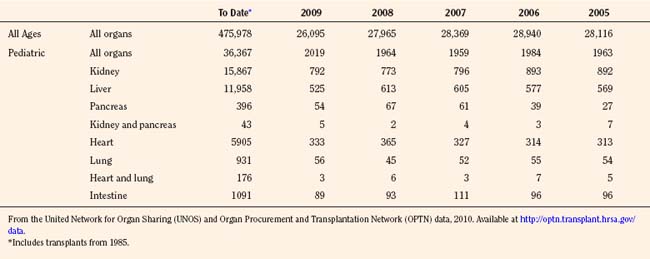
Since the inception of the United Network of Organ Sharing (UNOS) in 1987, nearly half a million solid organ transplants have been performed in the United States. Of these, 375,000 have been from deceased donors, or donation after cardiac death (DCD), and 103,000 have been from living donors (UNOS 4-2010) (Tables 28-1 and 28-2). The total number of pediatric transplants has reached nearly 37,000, and the total number of living donors is 9200 for all pediatric patients. In 2009, the yearly totals for pediatric organ transplants increased significantly for the first time in 6 years, to a total of 2019, from a low of 1964 transplants in both of the years 2005 and 2008. Unfortunately, the yearly total of pediatric living donors decreased from a decade high of 560 in 2001, to 387 in 2009. The incidence of end-stage organ disease increases with age, and children still account for only a small fraction (7.60%) of the total number of transplant recipients.
With the new changes, Organ Procurement Organization (OPO) members must submit data to the Organ Procurement and Transplantation Network (OPTN) under the directorship of the U.S. Department of Health and Human Services through the use of standardized forms and electronic databases. There are 11 OPTNs in the United States and Puerto Rico (Fig. 28-1). In addition to providing data on all deceased donors, living donors, potential transplant recipients, and actual transplant recipients, all OPOs must also submit of the total number of reported deaths by donor hospital.
FIGURE 28-1 A map of the 11 organ procurement and transplantation networks in the United States in 2010.
(From the United Network for Organ Sharing (UNOS) and Organ Procurement and Transplantation Network (OPTN) data, 2010. Available at http://optn.transplant.hrsa.gov/data.)
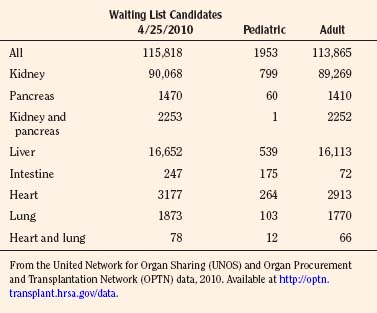
As of January 2010, there are 486 transplant centers or hospitals in the United States that operate one or more organ transplant programs. These centers house 1136 types of solid organ transplantation programs, managed by the 11 OPTNs (UNOS-OPTN, April 2010), as reported by the Health Resources and Services Administration of Health and Human Services. Data extracted from the UNOS database suggest that there are currently over 100,000 candidates awaiting transplantation. Table 28-3 demonstrates the numbers of pediatric and adult candidates awaiting organ transplantation by organ type. Pediatric patients outnumber these adults only in the category of intestinal transplantation.
The number of pediatric candidates awaiting organ transplantation pales in comparison with the total number of available organs. In fact, for the past 5 years, the total number of patients awaiting transplantation (~100,000) is one fifth of the total number of patients transplanted since 1985 (~500,000). Although organ transplantation procedures are relatively infrequent compared with the over 50 million surgical procedures performed per year in the United States, perioperative care for these patients has become highly specialized. Maintenance of physiologic homeostasis during the removal of a failed native organ and the subsequent allograft reperfusion period are challenging for both anesthesiologists and surgeons. This chapter focuses on the current body of knowledge for each type of major solid organ transplantation, with a specific focus on special considerations for the management of children. In addition, cellular transplantation, stem-cell transplantation, immunosuppression, complications, graft-versus-host disease, and posttransplant lymphoproliferative disease will be addressed. Information on artificial assist devices being used in the treatment of end-organ failure or as a bridge to transplantation, and information on xenotransplantation. See related video online at www.expertconsult.com.
Determination of brain death in children
The pediatric central nervous system (CNS) may be more resilient to certain forms of injury, a fact that should be considered when interpreting diagnostic evidence and confirming brain death in infants and children. Brain death is defined as the absence of cortical and cerebral function without preservation of brainstem function. When measured in this setting, cerebral blood flow (CBF) is absent. Publication of brain death criteria specifically addressing findings in infants and children has clarified most of the ambiguities created by age-related neurologic differences. The Task Force for the Uniform Determination of Brain Death in Children was assembled in 1987 (Report of Special Task Force, Pediatrics, 1988). Box 28-1 summarizes the currently accepted guidelines for determination of brain death in neonates, infants, and children.
Box 28-1 Guidelines for Brain Death in Children
If hypoxic encephalopathy is present, observation for 24 hours is recommended. This time may be reduced if an electroencephalogram shows electrocortical silence, or if a radionuclide study is negative for CBF. In patients with electrocortical silence, metabolic coma must be excluded. Detailed reviews of these guidelines and their applications have been published (Ashwal and Schneider, 1991; Ashwal, 1993; Koszer and Moshe, 2010). Prospective donors must fully meet the age-appropriate criteria and be declared legally brain dead before attempting to obtain consent for organ donation from their family. A physician must record the official time of death in the chart before transferring the donor to the operating room for the organ procurement procedure. Although the donor is officially dead, the care of an anesthesiologist is needed during these procedures to continue efforts to maintain cardiac output, at least until the viscera have been flushed with the cold preservative solution. At this point, ventilation can be discontinued and the responsibility of the anesthesiologist is concluded.
One area that remains particularly controversial is the appropriateness of organ donation from anencephalic newborns. This developmental anomaly results in absence of the cerebral cortex and upper brainstem, so these infants possess no potential for normal neurologic development. Despite the uniformly fatal outcome in these cases, such infants never meet the usual criteria for brain death (Baird and Sadovnick, 1984).
Donor strategies
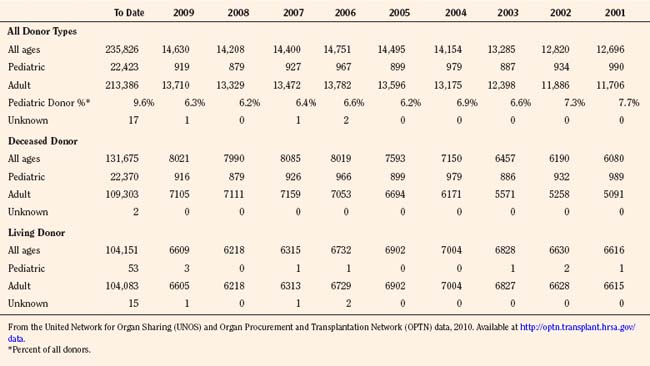
Organ donation in the pediatric population has decreased by almost 25% in the past 15 years, from 1201 in 1995, to 919 in 2009, with a low in 2008 of 879, the lowest number of pediatric donors since the inception of UNOS data collection (Table 28-4). Additionally, living donation has also decreased significantly in the past 5 years, a result of the ethics controversy and the complications that are now apparent with respect to living donation. The distribution of pediatric donor ages has remained consistent, with close to 60% being between the ages of 11 and 17 (Table 28-5). Although the number of adult donors is significantly greater than the number of pediatric donors, the percentage of recovered organs in the pediatric age range is generally greater than the percent of adult transplanted organs. For deceased kidney donors, 94.7% of the pediatric kidneys recovered were transplanted compared with 91.6% of the kidneys recovered from donors aged 18 to 49 years. There was a similar trend for pancreas donors (81.4% versus 72.2%), liver donors (96.0% versus 91.4%), and intestine donors (94.7% versus 85.7%). For heart and lung donors, the percentage of recovered organs transplanted was high, and it was similar for both pediatric donors and adult donors aged 18 to 49 years (98.9% versus 99.0% for heart and 98.1% versus 96.4% for lung) (OPTN/SRTR, 2010).
TABLE 28-4 Donors of Organs Recovered in the United States between January 1, 2001 and January 31, 2010
Recovery of organs from deceased donors requires continuing care of the patient’s preexisting condition, with a shift in primary emphasis from minimizing neurologic injury and preserving life to protection of specific organs. Principles of pediatric donor care are comparable with those of adults, but meticulous adjustments are necessary because of the special physiologic needs of children. The leading causes of death in children are asphyxia and trauma, so a history of cardiac resuscitation, hypotension, and hypoxemia is frequent in this donor population (Fischer-Froehlich et al., 2002).
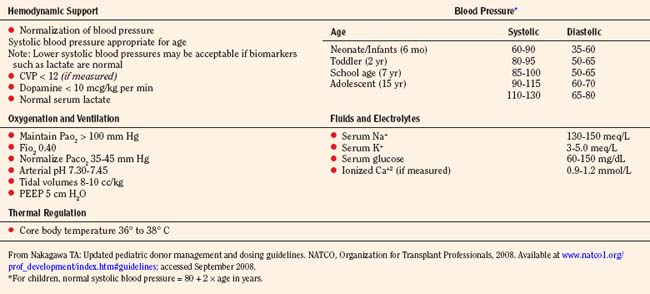
Pediatric donation management goals and dosing guidelines are available for reference from NATCO (Nakagawa, 2008b), the UNOS Critical Pathway for the Pediatric Organ Donor (www.unos.org), and the Canadian Council for Donation and Transplantation (www.ccdt.ca) (Shemie et al., 2006). In 2001, a Consensus Conference made specific recommendations for management of cardiac donors (Zaroff et al., 2002). See also Kutsogiannis (2006), Braunfeld (2004), Wood et al. (2004), and Ullah et al. (2006).
Ultimately, the management of the pediatric organ donation is dictated by regional standards of care and the physicians caring for the child. The primary responsibilities of the pediatric intensive care specialist and anesthesiologist are to anticipate the normal physiologic sequelae of brain death (Table 28-6), and to direct therapy toward normalizing gross alterations in physiologic and biochemical parameters.
TABLE 28-6 Normal Physiologic Sequelae of Brain Death
| Sequela | Cause | Management |
| Hypotension | Neurogenic shock, hypovolemia, hypothermia, electrolyte disorders, endocrine abnormalities, myocardial dysfunction | Mean arterial pressure > 60 mm Hg CVP 4 to 12 mm Hg PCWP 8 to 12 mm Hg SVR 800 to 1200 dyne/sec per cm5 Cardiac index > 2.4 L/min per m2 Inotropic support in order of preference (in mcg/kg per min): |
| Endocrine abnormalities | Disruption of the hypothalamic pituitary axis, resulting in adrenal insufficiency, hypothyroidism, or diabetes insipidus | Volume replacement; correct electrolytes; inotropic support Steroids: methylprednisolone (15 mg/kg) T3: 4-mcg bolus and 3 mcg/hr infusion Arginine vasopressin: 1-U bolus, then continuous infusion at 0.5 to 4 U/hr, titrated to a systemic vascular resistance of 800 to 1200 dyne/sec per cm5 Insulin: 1 U/hr minimum. Titrate to maintain blood sugar 120 to 180 mg/dL |
| Hypothermia | Loss of hypothalamic, neural, or endocrine regulation; resuscitation with cold fluids or blood products | Early aggressive warming to maintain temperature at >36° C At <30° C, may be unresponsive to ACLS, drug therapy, defibrillation, or pacing |
| Anemia | Hemorrhage, hemodilution | Transfuse, hemoglobin 7-10 g/dL |
| Coagulopathies | DIC, fibrinolysis, dilutional, hypothermia | Factor replacement, transfusion rewarming, early organ retrieval |
ACLS, Advanced cardiac life support; CVP, cerebrovascular pressure; DIC, disseminated intravascular coagulation; PCWP, pulmonary capillary wedge pressure; SVR, systemic vascular resistance.
From Rosengard B, Feng S, Alfrey E, et al: Report of the Crystal City meeting to maximize the use of organs recovered from the cadaver donor, Am J Transplant 2:701, 2002.
Organs from deceased donors may be injured or damaged from many things: the preexisting comorbid factors; hemodynamic instability (autonomic surge, hypotension, cardiac ischemia, metabolic acidosis, and use of vasopressors); endocrine, metabolic and electrolyte imbalances; hypoxemia (acute respiratory distress syndrome [ARDS]); coagulopathy; thrombocytopenia; renal failure; endotoxin release; and immune activation inherent in the brain death process (Jawan et al., 2002; Zaroff et al., 2002). The presence of brain death–related complications has been reported to have no effect on the number of organs donated if an aggressive donation management protocol is instituted (DuBose and Salim, 2008).
Organ Retrieval
Organ procurement is usually completed in 4 hours or less, depending on the operating team’s experience, the number of organs intended for retrieval, and the presence of variations in the vascular anatomy. In the face of worsening hypoxemia, coagulopathy, or refractory hypotension, the organ recovery surgery should be expedited to prevent warm ischemic injury to transplantable organs. The specific organs to be retrieved determine the type of fluid management, the ideal central venous pressure (CVP), the fraction of inspired oxygen (Fio2), and the choice of inotropic or vasopressor support and maximum allowable dosages. Management decisions depend on the organs being considered; for example, hemodynamic goals preferred by abdominal transplant surgeons occasionally differ from those of cardiothoracic transplant surgeons. Pediatric donation management goals are summarized in (Table 28-7) (Nakagawa, 2008b).
Strict asepsis is observed, prophylactic antibiotics are administered, and the donor is positioned and prepared surgically. If the corneas are to be harvested, the eyelids are taped shut and covered with cold saline compresses or ice packs for corneal protection. After 300 U/kg of heparin is administered and cardioplegia is achieved, the liver, intestines, pancreas, and kidneys are flushed with cold preservation solution, and sequential removal of organs can proceed. The spleen and omental lymph nodes are removed for tissue typing, and the aorta, inferior vena cava, and common carotid and iliac vessels are taken for vascular grafts. Anesthesia support of the organ donor is necessary until the proximal aorta is surgically occluded and in situ flushing of organs has begun. Subsequently, the ventilator should be disconnected and monitoring discontinued, as electrocardiographic (ECG) activity may persist for up to 70 minutes after cardiac arrest (Oaknine, 1975; Logigian and Ropper, 1985).
Because brain death results in the loss of central mechanisms that control the endocrine and autonomic nervous systems, cardiac death usually occurs within 48 to 72 hours despite maximal physiologic support. Up to 25% of potential brain-dead organ donors are lost each year in North America because of cardiovascular collapse (Jenkins et al., 1999). Hypotension and hemodynamic instability secondary to neurogenic shock, dysrhythmias, and hypovolemia, all resulting from the absence of brainstem function, should be anticipated and treated in all donors.
Intravascular Fluid Management
Brainstem injury produces a sequence of hemodynamic events, beginning with an increase in parasympathetic tone and evolving to a massive autonomic surge with hypertension (systolic blood pressure > 200 mm Hg) and tachycardia (>140 beats/min) (Audibert et al., 2006). The pathophysiology of neurocardiogenic injury involves an initial neural phase (catecholamine surge and mixed venous oxygen saturation [MVo2] supply-demand imbalance) within hours, followed by a humoral phase and release of inflammatory cytokines. Depletion of thyroid hormones and cortisol, and decreased coronary perfusion pressure may also impair myocardial function. Coronary vasoconstriction, subendocardial ischemia, focal myocardial necrosis, and endothelial injury are factors that contribute to accelerated cardiac allograft vasculopathy in the recipient (Segel et al., 2002; Szabo et al., 2002; Mehra et al., 2004). Systolic and diastolic dysfunction has been reported in 10% to 28% of pediatric donors and 71% of adult donors. It is reversible in the majority of cases (Banki and Zaroff, 2003). Despite normal or increased perfusion pressure, the resulting vasoconstriction may cause tissue ischemia that disrupts the production of ATP, generates oxygen free radicals, increases cytosolic calcium concentration, and activates various enzymatic cascades such as endonucleases and nitric oxide syntheses (Kunzendorf et al., 2002). A subsequent hypotensive phase caused by loss of autonomic regulation of the peripheral vasculature and unopposed vasodilation may further reduce oxygen supply to the tissues. Hypovolemia is seen with diuresis and fluid restriction during brain resuscitation, inadequate volume replacement after trauma, and diuresis resulting from diabetes insipidus or hyperglycemia.
Brain death is a dynamic inflammatory process. The activation of inflammatory mediators leads to a nonspecific immune response, which may be associated with accelerated acute graft rejection and poor long-term outcomes (Kunzendorf et al., 2002). In addition, a significant factor in organ injury after storage and cold ischemia is caused by reperfusion, initiated by leukocyte adhesion to endothelial cells and the production of oxygen-derived free radicals and peroxides.
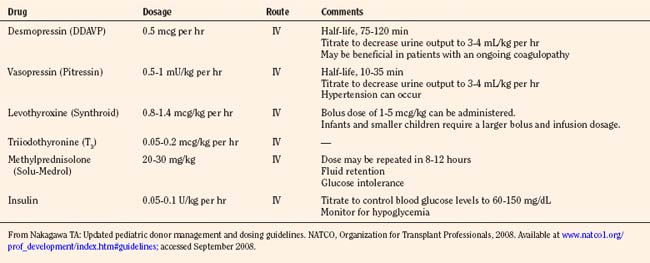
If the initial two-dimensional echocardiographic evaluation reveals that the left ventricular ejection fraction is less than 45%, management with invasive monitoring, fluids, inotropes, vasopressors, hormonal, replacement therapy (with hydrocortisone, levothyroxine, or triiodothyronine), and treatment of diabetes insipidus (with desmopressin [DDAVP] or vasopressin infusion) is strongly recommended (Table 28-8).
TABLE 28-8 Maintaining Mean Arterial Pressure in the Pediatric Organ Donor
| Hemodynamically Stable | Hemodynamically Unstable |
From Nakagawa TA: Updated pediatric donor management and dosing guidelines. NATCO, Organization for Transplant Professionals, 2008. Available at www.natco1.org/prof_development/index.htm#guidelines; accessed September 2008.
Hourly maintenance fluids are calculated according to the 4-2-1 rule; that is, for the first 10 kg, use 4 mL/kg; for the second 10 kg, use 2 mL/kg; when the weight is greater than 20 kg, use the weight in kilograms plus 40. Guidelines for transfusion therapy are listed in Table 28-9. Packed red blood cells (RBCs) are transfused to maintain the hemoglobin at 10 g/dL for unstable donors, with the lowest acceptable being 70 g/L. Platelets, International Normalized Ratio (INR), and partial thromboplastin time (PTT) have no predefined targets, with transfusion indicated in cases of clinically evident bleeding or coagulopathy. The recommended hemodynamic endpoints are mean arterial pressure (MAP), 60 mm Hg or greater; CVP and pulmonary capillary wedge pressure (PCWP), 12 mm Hg or less; systemic vascular resistance (SVR), 800 to 1200; cardiac index, 2.5 or greater; left ventricular stroke work index, 15 or greater; and dopamine dosage, 12 mcg/kg per minute or less (UNOS Critical Pathway for the Pediatric Organ Donor).
TABLE 28-9 Transfusion Therapy for the Organ Donor
| Component | Quantity (mL/kg) | Administration |
| Packed red blood cells | 10-15 | Administer over 2-3 hours. May be administered faster if hypotension or bleeding requires more aggressive correction of anemia. |
| Fresh frozen plasma | 10-15 | Administer over 1-2 hours. May be administered faster if correction of coagulopathy is associated with volume depletion or hypotension. |
| Cryoprecipitate | 5-10 or 1 unit for every 10 kg of body weight | Administer for hypofibrinogenemia. |
| Platelets | <15 kg: 10-20 >15 kg: single unit of platelets | Administer slowly over 2-3 hours. |
From Nakagawa TA: Updated pediatric donor management and dosing guidelines. NATCO, Organization for Transplant Professionals, 2008. Available at www.natco1.org/prof_development/index.htm#guidelines; accessed September 2008.
Cardiovascular Management
Hypotension in the potential organ donor most commonly results from hypovolemia (absolute or effective with a reduction in venous return), cardiac dysfunction, or arterial vasodilation from neurogenic shock (Table 28-10). The goals of hemodynamic support are to correct hypovolemia, to adjust vasoconstrictors and vasodilators to maintain a normal afterload, and to optimize cardiac output and maintain perfusion pressure gradients without relying on high dosages of β-agonists or other inotropes. Proposed adverse biochemical, histopathologic, and functional effects of catecholamine infusions on the myocardium include high-energy substrate depletion, intramyocardial noradrenaline depletion, and myocardial β-adrenoceptor down-regulation (Sakagoshi et al., 1992; Pinelli et al., 1995; Zaroff et al., 2002). With persistent hypotension despite volume replacement, vasopressor therapy should be initiated.
TABLE 28-10 Causes of Hypotension in the Potential Organ Donor
| Hypovolemia | Cardiac Dysfunction | Vasodilation |
| Initial injury Inadequate resuscitation Third spacing Decreased intravascular oncotic pressure after crystalloid resuscitation Dehydration from treatment of intracranial pressure Fluid restriction Urea Diuretics Mannitol Hyperglycemia-induced osmotic dieresis Diabetes insipidus Hypothermic “cold” diuresis Venodilation Loss of vasomotor tone and pooling in venous capacitance bed Rewarming of hypothermia | Preexisting disease Initial injury Myocardial contusion Pericardial tamponade Myocardial ischemia/infarct Brain death process Catecholamine damage Ischemia-reperfusion injury Metabolic depression Acidosis Hypothermia Hypophosphatemia Hypocalcemia Hypoxia Endocrinopathy of brain death Volume overload congestive heart failure Arrhythmias Catecholamines Ischemia Hypokalemia Hypomagnesemia | Spinal shock Catecholamine depletion Loss of vasomotor control and autoregulation “Relative” adrenal insufficiency of trauma or critical illness Endocrinopathy of brain death Acquired sepsis |
From Jalili M: Organ donor management: intensivist’s role. Pediatric Summit Powerpoint presentation, Los Angeles, March 2008. Available at www.onelegacy.org.
Dopamine up to 10 mcg/kg per minute is the drug of choice because the glomerular filtration rate (GFR) is increased as well as cardiac output while dilating the renal, mesenteric, and coronary vasculature. Inotropic agents should be titrated to maintain a normal blood pressure for the patient’s age (normal systolic blood pressure mm Hg = 80 + 2 × age in years). Blood pressure alone does not indicate adequate tissue perfusion. Therefore, serum biomarkers such as base deficit and lactate should be followed as inotropic support is titrated (Table 28-11).
TABLE 28-11 Inotropic Infusions
| Drug | Dosage |
| Milrinone (Primacor) | 0.25-0.75 mcg/kg per min IV Loading dose: 50 mcg/kg |
| Dopamine | 2-20 mcg/kg per min IV |
| Dobutamine (Dobutrex) | 2-20 mcg/kg per min IV |
| Epinephrine | 0.1-1 mcg/kg per min IV |
| Norepinephrine (Levophed) | 0.05-2 mcg/kg per min IV |
| Phenylephrine (Neosynephrine) | 0.1-0.5 mcg/kg per min IV Bolus: 5-20 mcg/kg |
| Vasopressin (Pitressin) | 0.3-2 mU/kg per min IV Note: Dosage is different for treatment of patients with diabetes insipidus |
From Nakagawa TA: Updated pediatric donor management and dosing guidelines. NATCO, Organization for Transplant Professionals, 2008. Available at www.natco1.org/prof_development/index.htm#guidelines; accessed September 2008.
Echocardiography should be performed early, followed by insertion of a pulmonary artery catheter, especially if the ejection fraction is less than 40% or the donor requires high-dosage inotropes. According to Zaroff (2004), ejection fraction is the most significant predictor of nonuse of potential donor hearts, with an odds ratio of 1.48 per 5% decrease in ejection fraction.
When dopamine is used for inotropic support in infants, dosages significantly higher than those needed in the adult may be required, presumably because of catecholamine receptor immaturity or deficiency (Kelly et al., 1984). At these higher dosages (15 mcg/kg per minute), no adverse effects on GFR or urine output are apparent (Outwater and Rockoff, 1984). Dopamine at α-agonist dosages has not been shown to influence outcomes in liver, kidney, or heart in pediatric donors (Finfer et al., 1996). There is evidence that dopamine may reduce allograft rejection, not only through support of blood pressure but possibly also through more complex immunomodulatory effects that inhibit expression of adhesion molecules, which are required for leukocyte migration into the graft to produce acute rejection (Carlos et al., 1997; Schnuelle et al., 1999).
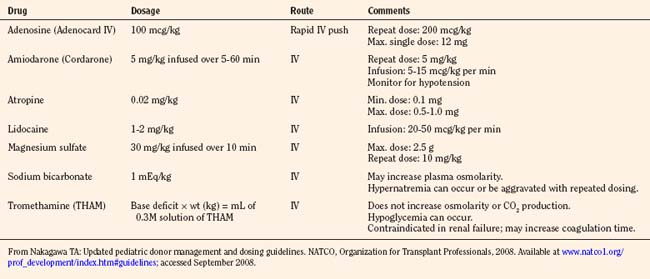
An infusion of an α-adrenoceptor vasoconstrictor, epinephrine or phenylephrine, may be useful to increase peripheral vascular tone, but they have the inherent risk of causing marked peripheral vasoconstriction and/or an increase in pulmonary artery pressure (PAP) or oxygen consumption. Jhanji and colleagues (2009) have shown norepinephrine to be protective of global oxygen consumption, without affecting the splanchnic circulation or producing significant deleterious renal effects. An infusion of vasopressin starting at 0.3 mU/kg per minute and titrated to the desired blood pressure, with a maximal infusion rate of 2 mU/kg per minute, may be efficacious in the setting of hypotension and low SVR, especially in combination with an inotropic infusion such as epinephrine. Vasopressin is not recommended as first-line therapy, as there are limited data available in children. Recent studies, however, suggest it is most efficacious when coupled with corticosteroids (Büchele et al., 2009). Dobutamine (IV, at 2 to 20 mcg/kg per minute) should be used when cardiac output is decreased because of reduced myocardial contractility and pulmonary hypertension. ECG abnormalities are common during the brain-death process, manifested as marked ST-T wave abnormalities, ischemic changes, inverted T waves, widened QRS complexes, and prolonged QT interval. In addition, atrial and ventricular arrhythmias and varying degrees of conduction abnormalities may occur. These arrhythmias usually result from autonomic instability (catecholamine storm and loss of the vagal motor nucleus) compounded by oxygenation, acid-base, temperature, and electrolyte (e.g., low magnesium and potassium) disturbances or increased intracranial pressure (ICP) (Cushing’s reflex) (Powner and Allison, 2006). The difficulty lies in differentiating these transient findings from those of catecholamine-induced myocardial injury or irreversible ####ischemia, which can produce global myocardial dysfunction. Bradycardia is not a problem unless it contributes to hypotension. It may be treated with any inotropic agent or temporary transthoracic or venous pacing.
Eventually, the heart stops. Despite all therapeutic efforts, the arrhythmias encountered are usually resistant to therapy (Table 28-12). Bradyarrhythmias leading to asystole rather than ventricular fibrillation (as seen in adults) are the terminal cardiac rhythms in pediatric patients. The propensity for this particular dysrhythmia may be related to the immature autonomic nervous system and small muscle mass in the pediatric donor (Walsh and Krongrad, 1983). In the event of a sudden cardiac arrest during the procurement procedure, cardiopulmonary resuscitation should be started to facilitate organ perfusion, and liver, pancreas, intestines, and kidney procurement should proceed rapidly with cross-clamping of the aorta at the diaphragm and infusion of cold preservation solution into the distal aorta and portal vein.
Pulmonary Management
All potential organ donors require mechanical ventilation, usually for a period of several days. Progressive pulmonary dysfunction may result from pulmonary contusions resulting from trauma, aspiration pneumonitis, fat emboli, and pulmonary edema (cardiogenic and neurogenic). Any of these may result in hypoxia, placing all perfuseable organs at risk for ischemia. In addition, infectious complications, oxygen toxicity, barotrauma or volutrauma, pulmonary embolism, and atelectasis may contribute to an increase in the alveolar-arterial oxygen gradient. High-dosage methylprednisolone administration has been shown to significantly improve oxygenation and increase donor lung recovery (Follette et al., 1998). The beneficial effects of steroids probably result from attenuation of the effects of proinflammatory cytokines released as a consequence of brain death (Glasser et al., 2001). Interleukin (IL)-8 expression and neutrophil infiltration of the donor lungs may result in impairment of graft oxygenation, development of severe early graft dysfunction, and early recipient mortality (Fisher et al., 2001). As the total lung water increases from a combination of pulmonary capillary leakage and disruption of the Starling forces in the lungs, pulmonary compliance decreases, with resultant impedance of alveolar gas exchange. Neurogenic pulmonary edema is best managed with positive end-expiratory pressure (PEEP), diuresis, and small volume fluid resuscitation. Lung-protective strategies include maintenance of an arterial saturation of 95% or a Pao2 greater than 100 mm Hg, with the lowest possible Fio2 setting preferably being no higher than 40%, and with Pao2/Fio2 greater than 300. Limiting high distention pressures in the lung during volume-controlled modes of ventilation using tidal volumes of 6 to 8 mL/kg, low peak inspiratory pressure (28 to 30 cm H2O), and avoiding PEEP in excess of 5 cm H2O is desirable. Paco2 should be normalized to 35 to 45 mm Hg, and serum pH to 7.35 to 7.45. The maintenance of a mild to moderate alkalemia has been reported by some to reduce the likelihood of ventricular fibrillation (programmed ventilator sigh) (Becker et al., 1981). Pulmonary hygiene, bronchodilators, and lung-expansion techniques every hour or two may help in preventing atelectasis and improve the quality of donor lungs. Recruitment maneuvers for oxygenation impairment include periodic increases in PEEP (up to 15 cm H2O) and sustained inflations (peak inspiratory pressure at 30 cm H2O for 30 to 60 seconds).
Endocrine Management
Once the diagnosis is confirmed, therapeutic intervention should consist of pharmacologic management to decrease urine output to 3 mL/kg per hour or less. Replacement of urine output with 0.2% one-fourth or 0.45% normal saline should be used in conjunction with desmopressin (intermittent IV DDAVP, 0.25 to 1 mcg every 6 hours) or vasopressin (0.5 to 0.7 mU/kg per minute IV to a maximum dosage of 2.4 U/hour) infusion to maintain serum sodium levels between 130 and 150 mEq/L. DDAVP is an analogue of arginine vasopressin, which is highly selective for the vasopressin V2 receptor subtype found in the renal collecting duct, and without the vasopressor activity in humans that is mediated by V1 receptors on vascular smooth muscle. DDAVP has a long half-life of 75 to 120 minutes, so an IV infusion may be discontinued 2 to 3 hours before organ recovery. Several mechanisms regulate the release of arginine vasopressin (AVP). Hypovolemia resulting from hemorrhage or any other cause results in a decrease in atrial pressure. Specialized stretch receptors in the atrial walls and large veins (cardiopulmonary baroreceptors) entering the atria decrease their firing rate when there is a fall in atrial pressure. Afferent nerve fibers from these receptors synapse in the nucleus tractus solitarii of the medulla, which sends fibers to the hypothalamus, a region of the brain that controls AVP release by the pituitary gland. Atrial receptor firing normally inhibits the release of AVP by the posterior pituitary. With hypovolemia or decreased AVP, the decreased firing of atrial stretch receptors leads to an increase in AVP release. Hypothalamic osmoreceptors sense extracellular osmolarity and stimulate AVP release when osmolarity rises, as occurs with dehydration. Finally, angiotensin II receptors located in a region of the hypothalamus regulate AVP. Therapy should always be guided by serum electrolyte and osmolality measurements made every 2 to 4 hours. Several investigators have demonstrated prolonged hemodynamic stability in donors, with the addition of an infusion of AVP to existing pressor support. Donors without clinically apparent DI may demonstrate a baroreflex-mediated defect of vasopressin secretion and pressor hypersensitivity to exogenous hormones. In these patients, low-dosage vasopressin significantly increases blood pressure (Chen et al., 1999). Absence of AVP may be a predominant contributing factor to the eventual cardiac arrest in all brain-dead patients.
Endocrine and Metabolic Functions
Despite disruption of the hypothalamic-pituitary-adrenal axis, hormone production from the anterior lobe of the pituitary gland persists in most brain-dead patients. The prevalence of anterior pituitary dysfunction is estimated at 30% (Schneider et al., 2005), with evidence of at least one abnormal hormonal function in 53% (Dimopoulou et al., 2004). More recently, Nicolas-Robin and colleagues (2010) noted in 31 brain-dead donors older than 18 years that the incidence of adrenal insufficiency was 87% and that the administration of 50 mg of hydrocortisone enhanced cardiovascular stability and decreased the requirement for norepinephrine. The incidences of hormonal reduction are estimated as follows: adrenal, 15%; thyroid, 5% to 15%; growth hormone, 18%; gonadal, 25% to 80%; and vasopressin, 3% to 37%. Endocrine failure is associated with basilar skull fracture, hypothalamic edema, prolonged unresponsiveness, hyponatremia, and hypotension (Powner and Allison, 2006).
Rapid depletion of vasopressin, cortisol, insulin, thyroxine (T4) and free triiodothyronine (T3) occurs in experimental animal models of brain death (Novitzky et al., 1984, 2006), but endocrine dysfunction in humans is usually manifested solely as DI (Wijdicks, 2001). Novitzky and others (1987) claim that true hypothyroidism exists, as evidenced by a decrease in free T3. They have shown that hormonal replacement therapy (T3, cortisol, and insulin with glucose) improves cardiac output and reduces the need for pressor support. Brain death is also associated with global mitochondrial dysfunction with a change to anaerobic metabolism and lactic acidosis. It leads to depletion of myocardial high-energy phosphates and cellular dysfunction. Thus, many organ procurement groups administer thyroid hormones to hemodynamically “rescue” unstable donors who show evidence of anaerobic metabolism or profound hypotension refractory to volume resuscitation and therapy with multiple vasopressors. In a landmark paper published in 1995, Wheeldon and colleagues (1995) showed that by using invasive hemodynamic monitoring and a standard donation management protocol (steroids, insulin, vasopressin, and T3), the Papworth Hospital Group in Cambridge, United Kingdom, was able to functionally resuscitate 92% of initially unacceptable donors into acceptable donors. Hormonal replacement therapy (methylprednisolone, T4) has been shown to decrease the need for inotropic support and improve metabolic stability in critically ill children with cessation of neurologic function and hemodynamic instability (Powner, 2001; Salim et al., 2001; Zuppa et al., 2004). Rosendale and colleagues (2003) compared non–hormone replacement with a three–hormone replacement protocol (methylprednisolone bolus and infusions of vasopressin and T3 or T4) in brain-dead donors, with 30-day mortality and early graft dysfunction used as measures of outcome. Multivariate results demonstrated a 46% reduced odds of death within 30 days and a 48% reduced odds of early graft dysfunction in the three–hormone replacement donor group.
An infusion of nitroglycerine or nitroprusside is occasionally recommended to prevent myocardial ischemia and the potential untoward renal effects of diminished renal perfusion with the use of vasopressin. Vasopressin supplementation (2 to 10 mcg/kg per minute continuous infusion) in a porcine model of brain-dead potential organ donors resulted in physiologic levels of the hormone, with normal plasma osmolarity and serum sodium levels and decreased urine output, potassium needs, and fluid requirements, without effects on peripheral vascular resistance and without microscopic evidence of organ ischemia (Blaine et al., 1984).
North American Transplant Coordinators Organization (Nakagawa, 2008a) currently recommends that hormonal replacement therapy (T4 or T3, steroids, insulin) be considered early in the course of pediatric donation management to improve donor heart function. Levothyroxine (Synthroid) is administered as a bolus dosage of 1 to 5 mcg/kg followed by an IV infusion of 0.8 to 1.4 mcg/kg per hour, with infants and smaller children requiring a larger bolus and infusion dosage. IV T4 has the disadvantage of a slower and unpredictable onset of action. Alternatively, T3 may be replaced as an infusion 0.05 to 0.2 mcg/kg per hour IV. Although T3 may rescue the donor heart, it may have detrimental effects, including tachycardia, arrhythmia, metabolic acidosis, and profound hypotension. Methylprednisolone (Solu-Medrol), 20 to 30 mg/kg IV bolus, is administered and may be repeated in 8 to 12 hours. An IV insulin infusion (0.05 to 0.1 units/kg per hour) is titrated to control blood glucose levels to 60 to 150 mg/dL, with monitoring for hypoglycemia (Table 28-13).
Electrolyte disturbances are routine, most being iatrogenic, resulting from treatment of the original injury or in response to physiologic perturbations during the brain-death process. Hypernatremia is inevitable as a result of DI, and a variety of strategies are used to treat elevated ICP. The liver is exquisitely sensitive to hypernatremia (>155 mEq/L) with increased levels correlating with primary graft loss after transplantation (Figueras et al., 1996). Serum potassium, magnesium, calcium, and phosphate levels are often depleted and require prompt replacement therapy. Hyperglycemia may be caused by glucose intolerance (steroids) or administration of large amounts of a dextrose-containing solution for fluid resuscitation.
Hypothermia
Although hyperthermia is common in the early phase of the sympathetic storm, the donor rapidly becomes hypothermic as a result of vasodilation and the loss of thermal regulation by the CNS. The patient becomes poikilothermic, with the body temperature determined by the environmental temperature. Although a mild degree of hypothermia may be beneficial to organ protection and preservation, the undesirable consequences of hypothermia, which are clinically significant, include diuresis and hypovolemia, hyperglycemia, coagulopathy, arrhythmias, myocardial depression, pulmonary hypertension, and eventually cardiac arrest (Reuler, 1978). Hypothermia further complicates the process of certification of brain death by causing the pupils to appear fixed and dilated. Active rewarming measures to maintain a core body temperature 36° to 38° C should be initiated early.
Organ Preservation
The field of organ preservation includes in situ core cooling, organ preservation fluids, and machine perfusion techniques. Traditionally, optimal organ preservation has been achieved by the combination of maximizing donor hemodynamics, using improved surgical procurement techniques with minimal dissection of vascular structures, cannulating the abdominal aorta for rapid in situ core cooling, removing abdominal organs en bloc with separation of graft components on the back table, employing cold storage techniques with a hyperkalemic hyperosmolar solution at a temperature of 4° C, and treating the donor or recipient (or both) pharmacologically. Most centers use static cold storage to preserve organs, and this is useful for younger donors with good-quality organs (Southard and Belzer, 1995; Marshall, 1997). With the introduction of extended donor criteria, the limitations of cold storage have probably been reached. Kidneys can be preserved for up to 72 hours but seldom do well in the recipient after 36 to 48 hours with cold ischemia. The heart and lungs should be transplanted within 4 to 6 hours, and the pancreas preferably within 6 to 12 hours.
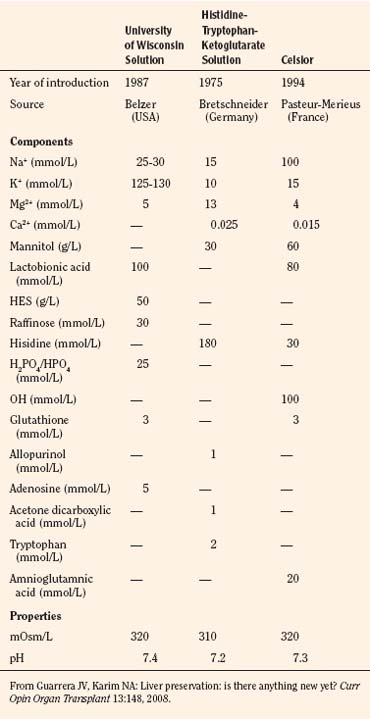
The preservation solution introduced by the University of Wisconsin (UW solution) in 1987 allowed extension of the safe preservation time of the donor livers from 8 to 24 hours (Jamieson et al., 1988) and kidneys for up to 72 hours. Iced storage and core cooling result in a slowing of cellular metabolism and energy consumption; glucose or hydrogen ion buffers prevent cellular acidosis; donor heparinization (30,000 U, or 300 U/kg) prevents microvascular thrombosis and promotes a more even organ flushing and reperfusion. Effective impermeants are saccharides and nonsaccharide anions. Molecular weight determines the effectiveness of saccharides to prevent cell swelling, with larger saccharides being more effective (Sumimoto et al., 1990; Hart et al., 2002). The UW solution, histidine-tryptophan-ketoglutarate (HTK), and Celsior are the primary solutions used currently for organ preservation (Table 28-14).
HTK, originally introduced for cardioplegia in 1980, has gained popularity in clinical transplantation for cold perfusion and organ storage since the U.S. Food and Drug Administration (FDA) approved its use for kidney and liver preservation (Lam et al., 1989). Early experiences with HTK have demonstrated that it compares favorably with the UW solution in kidney and liver organ preservation. Growing clinical experience with living donor liver transplantation has shown comparable clinical performances of UW solution and HTK (Lange et al., 1997; Testa et al., 2003). UW solution and HTK also show clinical equivalence for deceased donor liver transplants, although UW solution remains the gold standard for longer preservation times (Guarrera and Karim, 2008).
In the early 1970s, hypothermic machine perfusion (HMP) was used by many centers in the United States and Europe to preserve kidneys, allowing transportation to distant transplantation centers. Although modern HMP systems are smaller, lighter, and more sophisticated than the original machine used by Belzer and coworkers, the principles of HMP have not changed. Machine perfusion generates a controlled continuous or pulsatile recirculating flow of preservation solution at 0° to 4° C. This continuous flow allows complete perfusion of the organ, promoting a thorough washout of blood and subsequent tissue equilibration with the preservation solution. Beneficial effects of machine perfusion are a low incidence of delayed graft function, the possibility of real-time organ viability assessment, the ability to provide metabolic support during perfusion, and the potential to add pharmacologic agents to the perfusate. A meta-analysis reported by Wight and coworkers noted that HMP produced a 20% reduction in delayed graft function compared with cold storage (Guarrera and Karim, 2008). Most experience with HMP involves the kidneys (Henry, 1997), and clinical application of HMP in human liver transplantation has been limited to the recent pioneering work of Guarrera and colleagues (2004).
Living related organ donor
Over the past several years, a consistently widening gap has become evident between the demand for and supply of transplantable organs. At the end of 2009, there were 1907 pediatric transplant candidates on the waiting lists for various organs, out of a total of 113,702 candidates of all ages awaiting all organs. Of the pediatric candidates, approximately 50% were from 11 to 17 years old. This reflects a decline in the past 10 years and a decrease in the size of the liver and lung waiting lists, with the majority of children wait-listed for liver and kidney organs. The number of pediatric deceased donors over the past decade has steadily decreased (OPTN/UNOS, January 1988 to June 2010). Compared with adult deceased donors, pediatric deceased donors are more likely to donate a specific organ, as evident in data reported up to 2010: kidneys (92%), livers (87%), lungs (16%), heart (50%), pancreas (40%), and intestine (9%) (OPTN/UNOS, December 2009). With this serious shortage of donor organs of appropriate size and suitability for infants and small children, the waiting times for some organs has increased dramatically. For example, with only 20% of lungs from deceased donors meeting stringent donor criteria, the waiting period varies from 42% of pediatric patients waiting less than 1 year, to 15% listed for 5 or more years. This chronic shortage has prompted the development of programs for living related organ donation. The original concept was developed for renal transplantation in the early 1950s, and, currently in the United States, 25.2% of pediatric transplants are from living donors (OPTN/UNOS, 2009) (Table 28-15). Justification was relatively easy, as healthy relatives could “safely” donate one of their two kidneys without jeopardizing renal function in their remaining solitary kidney, and the results from living related pediatric renal transplantation have, until recently, been superior to deceased donor transplantation, at all ages. Later, techniques were developed to use one or more left-lateral segments of a parent’s liver, which would be suitable for a small child with chronic liver disease (Broelsch et al., 1991; Lang et al., 2004). Lessons learned from split or reduced-size deceased adult livers for implantation into children were used in the development of living related liver transplantation programs, which can accommodate large children and small adults using the right lobe of the liver. From 1987 to 2009 in the United States, 1264 of the total 3967 pediatric liver transplants were from living donors (see Table 28-15). With similar methods, living donor transplantation has been successfully developed for pancreas, lung (unilateral and bilateral lower lobes), and small bowel transplantation.
Living-related organ donation has, for the most part, been performed from parent to child. The use of children for living kidney donation remains highly controversial, and in general, most transplant programs do not use a donor younger than 18 years except in very limited circumstances, such as identical twins or an emancipated minor for his or her own child (Abecassis et al., 2000). Living-related donor procedures have several advantages, including the fact that they can be electively scheduled when the recipient is in optimal condition. However, with the exception of living related renal transplantation (which has excellent outcomes, with 1-year graft survival in children older than 1 year ranging from 94% to 96%), the long-term functional outcome of living-donor lung, pancreas, and small intestine transplants remains to be established because of the relatively small number of transplants performed. Currently, the risk for mortality and graft loss is less for children younger than 2 years if they receive a liver allograft from a living donor compared with a deceased donor (Reding et al., 2003). In addition, many troubling ethical issues arise, such as the risk to the donor and recipient, the validity of informed consent, and concerns about donor privacy and confidentiality. Uncomplicated unilateral nephrectomy has an exceedingly low mortality (less than 0.1%), but the same is not true for right lobe liver donors (0.2% to 1%, or 4 to 10 of 2000 cases), left segment/lobe liver donors (0.06% to 0.2%, or 2 to 6 of 3300), and right lobectomy of the lung, less than 5% mortality (Hayashi and Trotter, 2002; Trotter et al., 2002). Additionally, Hashikura and colleagues (2009) reported an 8.4% complication rate in 299 donors undergoing living related liver donation. Postoperative donor complications included biliary complications (3.0%), reoperation (1.3%), severe aftereffects in two cases (0.06%), and death (apparently related to donor surgery) in one donor (0.03%). The incidences of postoperative complications in left- and right-lobe donors were 8.7% and 9.4%, respectively.
In addition, there have been increasing interest and use of DCD, with the hope of expanding this pool from 200 to 1000 donors per year in the United States. Although pediatric donors constitute approximately 20% of the total DCD donor pool, very few of the kidneys recovered are allocated to pediatric recipients (OPTN/UNOS, 2009). This practice may reflect concerns about long-term graft function, given the limited outcomes data available.
Immunosuppression
Immunosuppression Medications
Corticosteroids
Corticosteroids were the initial mainstay of therapy for organ transplantation, but their use is on the decline. They are particularly effective in both the prevention and treatment of acute rejection episodes. Their predominant mechanisms of action include the inhibition of IL-1 and IL-2 production, suppression of helper and suppressor T cells, suppression of cytotoxic T cells, and reduction in the migration and activity of neutrophils (Cohen, 2002). The long-term use of corticosteroids has been questioned because of their adverse side effects, particularly in the pediatric population. The trend during the past decade and a half is to use fewer steroids for maintenance therapy (Margarit et al., 1989; Ascher, 1995). Many centers do not now use steroids as part of their long-term regimens. The first report of steroid withdrawal after transplantation was published in 1989 (Margarit et al., 1989). Everson and associates (1999) published a literature review, which demonstrated that greater than 50% to 85% of patients can be withdrawn from steroids without changes in acute rejection episodes, patient survival, or graft survival rates (Stegall et al., 1997). This percentile difference depends on the antirejection agent used for maintenance therapy (e.g., tacrolimus versus cyclosporine or serolimus). Steroid withdrawal is preferable, as it is associated with significantly less hypertension, hypercholesterolemia, and diabetes mellitus, and, moreover, it is less likely to result in cushingoid features and delayed development and obesity in the pediatric patient. Despite controversy over their use in maintenance therapy, corticosteroids remain the first-line agents for the treatment of acute rejection. The response rates vary depending on the organ system and whether the patient is maintained on cyclosporine or tacrolimus (U.S. Multicenter FK506 Liver Study Group, 1994).
Azathioprine
Azathioprine, the imidazole derivative of 6-mercaptopurine, was introduced in the late 1950s and early 1960s. Its mechanism of action is the inhibition of differentiation and proliferation of T and B lymphocytes by blocking DNA and RNA synthesis. The effect is a reduction in the numbers of circulating white cells, both lymphocytes and granulocytes. One notable advantage of this agent is its steroid-sparing effect, allowing lower dosages of steroids to be used with equal efficacy and fewer side effects. The use of this agent is limited, however, by side effects, particularly hematologic and gastrointestinal (GI). Its use may result in as many as 50% of patients exhibiting bone marrow suppression and a profound leukopenia or thrombocytopenia (Cattral et al., 2000). Gastrointestinal side effects include pancreatitis, nausea, vomiting, and hepatotoxicity. Patients are at increased risk for opportunistic infections. An increased risk for malignancy, especially lymphoma, however, remains controversial. The primary pathway of metabolism is inactivation by xanthine oxidase, an enzyme blocked by allopurinol. The recommended dosage of azathioprine is 1 to 2 mg/kg per day. Plasma levels are not monitored for this agent.
Mycophenolate Mofetil
Mycophenolate mofetil (MMF) (CellCept) is a morpholinoethyl ester of mycophenolate. It is well absorbed orally and hydrolyzed to its active form, mycophenolic acid, which acts by competitively inhibiting inosine monophosphate dehydrogenase and hence blocks de novo synthesis of purines, primarily guanine. Lymphocytes, unlike other rapidly replicating cells, depend entirely on this pathway for purine synthesis. Hence, the primary mechanism of action of MMF is the selective inhibition of lymphocyte proliferation. This drug is one of the newer agents introduced in the past 10 years and has been shown to be more efficacious than azathioprine in combination therapy (Fisher et al., 1998). There is also some experimental evidence to suggest that MMF may reduce the risk for chronic allograft rejection, which has been attributed to its antiproliferative activity against B lymphocytes and arterial smooth-muscle cells (Azuma et al., 1995; Schmid et al., 1995). The most common side effects are GI, including nausea, abdominal pain, anorexia, gastritis, and diarrhea. Diarrhea affects as many as 30% of patients but usually responds to a decrease in dosage. MMF also causes leukopenia and thus should be avoided in combination with azathioprine. The usual dosage is 1 to 3 g/day orally in two divided doses. Like azathioprine, blood levels are not monitored with this agent. The antivirals acyclovir and ganciclovir increase MMF levels, and antacid decrease the absorption of MMF (Cohen, 2002).
Calcineurin Inhibitors
Cyclosporine
Cyclosporine is a neutral lipophilic cyclic endecapeptide extracted from the fungus Tolypocladium inflatum in the early 1970s. Cyclosporine is poorly absorbed from the GI tract, and there is considerable variation in its bioavailability, as biliary output is essential for its absorption. Neoral (Novartis Pharmaceutical Corp., East Hanover, NJ), the microemulsion formula of cyclosporine, is much less dependent on bile production and flow than Sandimmune (Novartis Pharmaceuticals Corp.), the standard preparation. Neoral gives a more consistent level of cyclosporine and is associated with less evidence of rejection (Graziadei et al., 1997; Pinson et al., 1998), so it has replaced Sandimmune in most major transplant centers worldwide.
Cyclosporine is metabolized by the cytochrome P450 3A system (Cantarovich et al., 1998). Agents that induce these enzymes increase the metabolism of cyclosporine, resulting in lower blood levels, whereas agents that inhibit P450 activity result in higher circulating cyclosporine levels. In addition, cyclosporine can interfere with the metabolism of other medications. Digoxin, lovastatin, and prednisolone can have significantly decreased clearance with resultant toxicity. The side effects associated with cyclosporine are numerous. Nephrotoxicity is one of the most significant, and it may involve acute or chronic renal pathologic alterations. The acute nephrotoxicity, which often resolves on reducing or discontinuing the medication, probably results from afferent arteriolar vasoconstriction with resulting decreased GFR. The chronic nephrotoxicity, which does not appear to be reversible, is associated with arteriolar hyalinosis, tubular vacuolization, interstitial nephritis, and cortical atrophy. Neurotoxicity is another common side effect, seen in as many as 50% of patients. This can range from headaches and tremors to seizures and coma. Because of variations in absorption and metabolism, cyclosporine levels tend to fluctuate. Dosages should be determined on the basis of the formulation used (i.e., Neoral versus Sandimmune) and serum or blood levels. Techniques to measure cyclosporine levels include radioimmunoassay and high-pressure liquid chromatography. The levels can be measured in whole blood, serum, or plasma. Most centers use whole blood trough levels by the radioimmunoassay technique. A level of 100 to 200 ng/mL is generally desirable in the first 3 to 6 months after transplantation, depending on the organ transplanted. The level can usually be maintained at lower levels thereafter, depending on the organ transplanted.
Tacrolimus
The calcineurin inhibitor tacrolimus, initially researched and brought to the market under the name FK-506, is a novel immunosuppressant that was isolated from Streptomyces tsukubaensis in 1985 (Kino et al., 1987; Starzl et al., 1989). Tacrolimus (FK506, Prograf) shares many similarities with cyclosporine, including its basic mechanism of action and metabolism by cytochrome P450 3A, in addition to its side-effect profiles. However, significant differences between these drugs have been elucidated in the past decade. Tacrolimus is 10 to 100 times more potent than cyclosporine, it possesses hepatotrophic properties, and it does not rely on bile for its absorption. Renal impairment, neurotoxicity, and hypertension occur with similar frequencies in both cyclosporine- and tacrolimus-based regimens. Tacrolimus is associated with more hyperglycemia. Reportedly, as many as 20% of tacrolimus recipients become insulin-dependent diabetics because of the agent’s direct effect on pancreatic beta cells and peripheral insulin receptors. Tacrolimus appears to have a higher incidence of posttransplant lymphoproliferative disease. Considering that accelerated atherosclerosis is an important issue after solid organ transplantation, it is important to note that tacrolimus produces less hyperlipidemia and thus a less adverse cardiovascular risk profile than cyclosporine. Statistically significant improvements have been seen in total cholesterol, low-density lipoprotein cholesterol, and triglyceride levels when patients were switched from cyclosporine to tacrolimus (Manzarbeitia et al., 2001). Tacrolimus levels are usually measured in whole blood specimens by enzyme immunoassay techniques. Drug levels of 5 to 15 ng/mL are desirable in the first few months after transplantation, depending on the organ system transplanted.
Efficacy of Cyclosporine and Tacrolimus
After its introduction, cyclosporine became the drug of choice for the prevention of allograft rejection. Tacrolimus, initially reserved for use as rescue therapy in refractory rejection, has now become a first-line immunosuppressive agent in many centers worldwide. Large multicenter U.S. and European randomized clinical trials have been performed to compare tacrolimus and cyclosporine (Sandimmune) for primary and maintenance immunosuppression (European FK506 Multicenter Liver Study Group, 1994; U.S. Multicenter FK506 Liver Study Group, 1994; Wiesner, 1998

Full access? Get Clinical Tree