Anesthesia for Laparoscopic and Robotic Surgeries
Girish P. Joshi
Anthony Cunningham
Key Points
Related Matter
Laparoscopic
Introduction
Table 43-1. Advantages of Minimally Invasive Surgery | |
|---|---|
|
This chapter discusses the anesthetic management of adult patients undergoing laparoscopic and abdominal robotic surgeries. However, endoscopic and robotic cardiac and thoracic surgeries (e.g., video-assisted thoracic surgery [VATS]) that are increasingly performed are discussed elsewhere (Chapter 37).
Surgical Techniques
it has not been accepted in routine clinical practice because it increases operating times and surgical costs without improving clinical outcomes.13
Table 43-2. Potential Complications During Laparoscopy | |
|---|---|
|
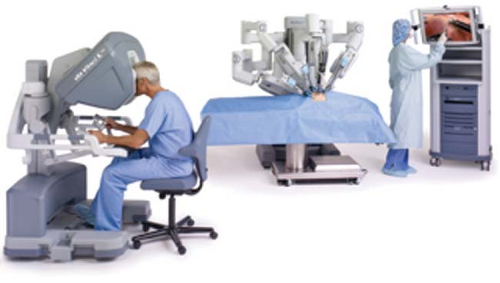 Figure 43.1. A control console where the surgeon is stationed and operates the robotic arms and camera. © 2012 Intuitive Surgical, Inc. |
The initial access necessary for CO2 insufflation could be achieved either through a blind insertion of a Veress needle that consists of a blunt-tipped, spring-loaded inner stylet and sharp outer needle through a small subumbilical incision or a trocar inserted under direct vision. The open insertion of the trocar using a minilaparotomy approach guarantees safe creation of pneumoperitoneum and avoids the dangers of blind insertion.
Upon confirmation of appropriate placement, a variable flow electronic insufflator that automatically terminates gas flow at a preset intra-abdominal pressure (IAP) is used to achieve pneumoperitoneum. It is standard of care to maintain the IAP below 15 mm Hg, because higher pressures can have significant physiologic consequences and can increase the incidence of intraoperative complications. An access port is then inserted in place of the needle to maintain insufflation during the procedure. A video laparoscope, inserted through the port, allows visualization of the operative field. Additional access ports are inserted through a number of small skin incisions, which allow the introduction of surgical dissection and suction instruments. The secondary ports are placed under direct vision, preferably with the help of transillumination of the abdominal wall to identify superficial abdominal wall vessels.
The Da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, California) is the most common robotic platform used currently. It consists of three components: a control console where the surgeon sits and operates the robotic arms and camera (Fig. 43-1), an equipment tower that includes an optical system, and the patient side cart that includes robotic arms.3,4,6 Similar to a laparoscopic procedure, robotic surgery involves development of pneumoperitoneum and placement of a video camera (a high-definition three-dimensional vision system) and ports. This is followed by placement of the robotic arms, a crucial and tedious part of the procedure (Fig. 43-2). An assistant is at the patient side for suctioning, retraction, and passage of suture or sponges.
Patient position during minimally invasive surgery varies significantly based on the surgical procedure. Patients undergoing upper abdominal procedures require a reverse Trendelenburg (head-up) position, while those undergoing lower abdominal procedures require Trendelenburg (head-down) position. The head-up or head-down position can be steep. In addition, the operating tables may be rotated laterally (right or left lateral) to further facilitate surgical exposure. Also, patients undergoing pelvic surgery (e.g., radial prostatectomy and hysterectomy) may be placed in a lithotomy position. Patients undergoing urologic surgery, particularly renal procedures, may be placed in lateral or semilateral positions combined with a flexion (i.e., jackknife) position. The considerations related to patient positioning are discussed in Chapter 28.
Physiologic Effects
The physiologic consequences of laparoscopy can be complex and depend on the interactions between the patient’s pre-existing cardiopulmonary status and surgical factors such as the magnitude of IAP, degree of CO2 absorption, alteration of patient position, and the type of surgical procedure.2,14,15 In addition, the anesthetic technique may influence the physiologic changes; however, these effects may be minimal with modern anesthetic techniques. Of note, physiologic changes are well tolerated by most healthy patients; however, they could have adverse consequences in patients with limited cardiopulmonary reserve.
Cardiovascular Effects
The changes in the cardiovascular function during laparoscopy are due to the mechanical and neuroendocrine effects of pneumoperitoneum and the effects of absorbed CO2 and patient positioning as well as patient factors such as cardiopulmonary status and intravascular volume (Table 43-3). The induction of pneumoperitoneum in the supine position (rather than head-down position) and limiting the IAP to 12 to 15 mm Hg minimize the alterations in cardiovascular function during laparoscopy.2,14
in SVR. In addition, compression of the arterial vasculature from increased IAP may also lead to an increase in SVR. These neuroendocrine and mechanical responses supersede the hypercapnia-induced arteriolar dilation and decrease SVR. The increase in SVR may increase the myocardial wall tension and, thus, may increase the myocardial oxygen demand. However, myocardial ischemia, as suggested by electrocardiogram-ST–segment changes, is not observed.17
Table 43-3. Hemodynamic Effects of Minimally Invasive Surgery | |
|---|---|
|
The changes in cardiac filling pressures and volumes during laparoscopy appear to be complex. Increased cardiac filling pressures may reflect increased intrathoracic pressures caused by pneumoperitoneum and increased sympathetic output due to hypercapnia from CO2 absorption and surgical stress. However, cardiac filling pressures may not always reflect cardiac filling volumes. Increased IAP may compress venous capacitance vessels, causing a decrease in preload (cardiac filling volume), particularly in hypovolemic patients. In contrast, compression of the abdominal organs (e.g., liver and spleen) caused by increased IAP may increase intravascular volume, which may increase cardiac filling, particularly if the patient is placed in a head-down position.18 Overall, the cardiac filling pressures and volumes increase, but minimally.
In healthy patients, the changes in cardiac index (CI) appear to be phasic with initial reduction after induction of pneumoperitoneum and subsequent recovery within 10 to 15 minutes. Overall, the changes in CI in healthy patients are minimal. However, in patients with severe cardiac dysfunctions, there may be a significant reduction in CI and significant hemodynamic deterioration.19 Although reduction in CI parallels the time course of increase in SVR, the cause–effect relationship between SVR and CI is unclear. In addition, significant hypercapnia and associated respiratory acidosis may decrease myocardial contractility and lower the arrhythmia threshold. Hypercarbia can cause pulmonary vasoconstriction, which may be deleterious in patients with pulmonary hypertension or right ventricular dysfunction.
In the elderly, with significant coexisting cardiopulmonary disease, pneumoperitoneum and head-down position cause several hemodynamic changes.22 Induction of pneumoperitoneum significantly increased SVR accompanied with a significant reduction in CI and ejection fraction (EF). However, the left ventricular workload remained unchanged. Upon placement in the head-down position, the cardiac preload, as determined by the left ventricular end-diastolic area, increased and CI and EF improved.22 Oxygenation and ventilation remained unchanged, and no patients exhibited electrocardiogram signs of myocardial ischemia. Release of pneumoperitoneum resulted in a significant decrease in SVR and increased CI and left ventricular systolic work index.
The type of surgical procedure may also influence the degree of hemodynamic derangement. Surgical disruption of the esophageal hiatus during laparoscopic fundoplication may increase mediastinal and pleural pressures, resulting in a significant reduction in CI.23,24 Patients undergoing endoscopic radical prostatectomy in the Trendelenburg position did not experience hemodynamic changes, despite prolonged duration (average 4 hours) of pneumoperitoneum.25
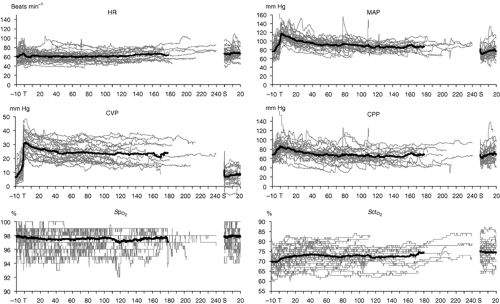
The hemodynamic changes that occur during abdominal robotic surgery appear to be similar to those observed during laparoscopic surgery. Most of the studies evaluating such changes are performed in patients undergoing prostatectomy with steep head-down position. Peritoneal insufflation and steep (40 degrees) head-down position during robotic-assisted prostatectomy increase SVR and MAP, while other hemodynamic variables remain in acceptable limits26,27 (Figs. 43-3 and 43-4). A recent study found that although cardiac filling pressures were increased, the cardiac performance (stroke volume, cardiac output, and mixed venous oxygen saturation as well as
echocardiographic cardiac dimensions) was maintained during robotic-assisted prostatectomy with patients in 45-degree head-down tilt and pneumoperitoneum with IAP 12 mm Hg28 (Fig. 43-5). Overall, robotic surgery appears to be well tolerated in a healthy population. However, the physiologic changes in the elderly or in patients with impaired cardiopulmonary reserve undergoing robotic prostatectomy remain unknown.
echocardiographic cardiac dimensions) was maintained during robotic-assisted prostatectomy with patients in 45-degree head-down tilt and pneumoperitoneum with IAP 12 mm Hg28 (Fig. 43-5). Overall, robotic surgery appears to be well tolerated in a healthy population. However, the physiologic changes in the elderly or in patients with impaired cardiopulmonary reserve undergoing robotic prostatectomy remain unknown.
Regional Perfusion (Splanchnic, Renal, Cerebral, Intraocular)
minimal access surgery (Table 43-4). However, the clinical consequences of these changes depend largely on the patient’s pre-existing status.
Table 43-4. Regional Circulatory Changes During Laparoscopy | |
|---|---|
|
The direct mechanical and neuroendocrine effects of pneumoperitoneum can decrease splanchnic circulation, causing reduced total hepatic blood flow and bowel circulation. However, these effects may be counterbalanced by the direct splanchnic vasodilatation caused by hypercapnia. Notwithstanding occasional reports of mesenteric ischemia following laparoscopy, the effects of pneumoperitoneum on the splanchnic circulation are not clinically significant.
The mechanical compressive and neuroendocrine effects of pneumoperitoneum may account for reduction in renal blood flow, glomerular filtration, and urine output (Table 43-5).29,30 However, the urine output generally normalizes following pneumoperitoneum deflation with no consequent renal dysfunction. Nevertheless, there may be clinical implications in critically ill patients and those with renal dysfunction undergoing extensive laparoscopic procedures requiring prolonged pneumoperitoneum.
An increase in PaCO2 during steep Trendelenburg positioning can increase cerebral blood flow and intracranial pressure with implications for patients with intracranial mass lesions. Therefore, maintenance of normocarbia is essential for preservation of cerebrovascular homeostasis.31 However, cerebral oxygenation and cerebral perfusion remain within safe limits during combined pneumoperitoneum and Trendelenburg position.27,32,33
Table 43-5. Renal Function During Laparoscopy | |
|---|---|
|
Choroidal vasodilatation and an increase in intraocular pressure may occur during CO2 pneumoperitoneum and steep head-down position.33 Intraocular pressure increased significantly during robotic-assisted radical prostatectomy with steep head-down position.33 Multivariate analysis suggested that the predictors of IOP include duration of surgery and end-tidal CO2 (ETCO2).
Respiratory and Gas Exchange Effects
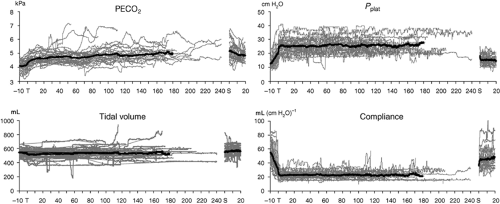
The CO2 insufflated into the peritoneal cavity is absorbed and causes hypercarbia. The absorption of gas from the peritoneal cavity depends on its diffusivity, the absorption area, and vascularity of insufflation site. Carbon dioxide absorption is greater during extraperitoneal (e.g., pelvic, hernia repair, and adrenorenal surgeries) insufflation than during intraperitoneal insufflation (e.g., cholecystectomy).34 The CO2 absorption reaches a plateau within 10 to 15 minutes after initiation of intraperitoneal insufflation and thus is not influenced by the duration of surgery.35 However, it continues to increase progressively throughout extraperitoneal CO2 insufflation.
Table 43-6. Pulmonary Changes During Laparoscopy | |
|---|---|
|
A recent study found that pH decreased during laparotomy open procedures and laparoscopic procedures with CO2 pneumoperitoneum. However, reduced pH during the pneumoperitoneum was due to an increase in PaCO2 and promptly returned to a normal value after the desufflation of the abdomen. In contrast, reduction in pH after laparotomy was from metabolic factors and persisted for approximately an hour postoperatively.36
A recent animal study found that the improved arterial oxygenation and gas exchange after induction of pneumoperitoneum was due to improved ventilation–perfusion matching caused by redistribution of perfusion away from the collapsed lung regions. This was probably caused by enhanced hypoxic pulmonary vasoconstriction possibly mediated via increased arterial CO2.37
During robotic-assisted hysterectomy and prostatectomy performed under steep (40 degrees) head-down position, the changes in dead-space ventilation and venous admixture appear to be small.38 Another study in patients undergoing robotic prostatectomy also found minimal changes in respiratory parameters.27 However, the arterial end-tidal CO2 gradient increased after 120 minutes. Therefore, ETCO2 values may underestimate arterial CO2 levels, and maintaining ETCO2 between 25 and 35 mm Hg will result in PaCO2 levels of 35 to 45 mm Hg. Similarly, the institution of pneumoperitoneum (IAP of 12 mm Hg) and 45-degree head-down positioning resulted in decreased lung compliance by 40%.28 The ventilation–perfusion distribution did not differ significantly from baseline measurements, and oxygenation actually improved, probably due to optimization of intraoperative ventilation.28
Anesthetic Management
Induction of Anesthesia and Airway Management
Because of its unique recovery profile, propofol is considered the sedative–hypnotic drug of choice for induction of anesthesia. Propofol also offers an advantage over other intravenous anesthetics because of its antiemetic properties and associated euphoria on emergence. Tracheal intubation and controlled mechanical ventilation comprise the accepted anesthetic technique to reduce the increase in PaCO2 and avoid ventilatory compromise from pneumoperitoneum and position changes. Although the laryngeal mask airway (LMA) has been used during short pelvic laparoscopic procedures, this evidence cannot be extrapolated to procedures requiring high IAP, steep head-down position, and upper abdominal laparoscopy as well as in patients at increased risk of regurgitation.42,43
Maintenance of Anesthesia
Maintenance of anesthesia with the newer inhaled anesthetics (i.e., desflurane or sevoflurane) remains the mainstay of modern anesthesia practice, probably because of the ease of titratability.39,44 In addition, inhaled anesthetics exert some neuromuscular blocking effect. Furthermore, inhalation anesthesia may provide faster emergence as compared to total intravenous anesthesia (TIVA) with propofol. However, propofol-based TIVA is associated with a lower risk of postoperative nausea and vomiting (PONV), but its cost and apparent complexity (i.e., need for infusion and difficulty in titration) deter some practitioners.44,45 Of note, except for patients with very high risk of PONV, the incidence of PONV with TIVA appears to be similar to that with inhalation anesthesia combined with prophylactic antiemetics.44

Full access? Get Clinical Tree