19 Anemia
The traditional approach for the management of anemia in the ICU has been the administration of packed red blood cell (PRBC) transfusions. On average, about 40% of ICU patients are transfused (a mean of 5 units of PRBCs) in response to a mean pretransfusion hemoglobin (Hb) concentration of 8.5 g/dL.1 Over the last decade, several studies have shown that PRBC transfusion is independently associated with worse clinical outcomes, independent of the degree of anemia or the severity of illness. Myriad complications resulting from PRBC transfusion are increasingly being recognized, and the scarcity of blood (expected annual shortfall of 4 million units by the year 20302) and economic impact of PRBC transfusion (approximately $270 per unit transfused3) have prompted a paradigm change for managing anemia in the ICU.
 Epidemiology
Epidemiology
Anemia is defined as Hb level less than 13 g/dL for adult males and less than 12 g/dL for adult nonpregnant females.4 Using this definition, more than 60% of all patients are anemic at admission, and the majority of those with normal Hb levels at admission become anemic while in the ICU.5,6 Given enough time, virtually all patients will become anemic during their ICU stay. In the anemia and blood transfusion in critically ill patients study (the ABC trial), 63% of patients had Hb levels below 12 g/dL, and 29% had Hb levels below 10 g/dL.5 Similarly, in the CRIT study, mean Hb level at baseline was 11 g/dL.6
The most frequent strategy for treatment of anemia is the transfusion of PRBC. As a consequence, more than 14 million units are transfused annually in the United States.7 In patients with malignancy as their admission diagnosis, the prevalence and incidence of anemia are 68% and 47%, respectively.8 Each day in the ICU increases the chance of being transfused by about 7%.9
 Etiology
Etiology
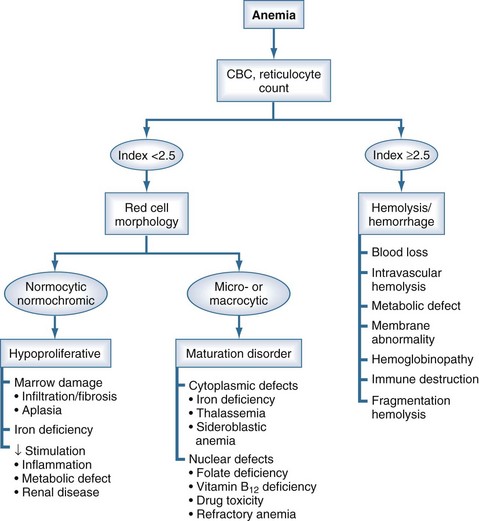
Figure 19-1 Physiologic Classification of Anemia.
(Adapted from Fauci AS, Kasper DL, Braunwald E, et al, editors. The physiologic classification of anemia. In: Harrison’s Principles of Internal Medicine, 17th ed., online: http://www.accessmedicine.com. Copyright © The McGraw-Hill Companies, Inc. All rights reserved.)
Blood loss due to phlebotomy is an often unrecognized yet significant cause of anemia in the ICU, where patients are phlebotomized on average 4.6 times a day, with removal of 40 to 60 mL of blood daily.5,6,10,11 The volume of blood removed varies with the test being ordered, but average volumes typically drawn are presented in Table 19-1. The presence of an arterial line further increases the phlebotomized blood volume.11 Approximately half of all patients are transfused as a direct result of phlebotomy.11
TABLE 19-1 Average Volumes of Blood Drawn for Diagnostic Testing89
| Arterial blood gas | 2 mL |
| Chemistry | 5 mL |
| Coagulation studies | 4.5 mL |
| Complete blood counts | 5 mL |
| Blood culture | 10 mL |
| Drug levels | 5 mL |
| Standard discard amount | 2 mL |
Although rare since the advent of effective GI prophylaxis, GI bleeding can be a serious problem in the ICU. The overwhelming majority of critically ill patients demonstrate evidence of mucosal damage within the first 24 hours of admission. Overt anemia occurs in 5% of patients with stress-related GI bleeding, and clinically important bleeding necessitating transfusion is observed in 1% to 4% of critically ill patients.12 Bleeding secondary to erosive gastritis is predominantly seen in patients on mechanical ventilation, patients with coagulopathy, patients with head injury, and/or patients receiving corticosteroids.13
Reduced erythropoietin production is a key feature of anemia of critical illness, a distinct clinical entity similar to anemia of chronic disease. This blunted erythropoietic response to low Hb concentration in the face of apparently adequate iron stores is due to a failure to produce appropriate levels of erythropoietin.14,15 Blunted erythropoietin production in critically ill patients is probably mediated by proinflammatory cytokines such as tumor necrosis factor (TNF), interleukin (IL)-1, and IL-6, which down-regulate expression of the gene encoding erythropoietin.16 IL-6 inhibits renal erythropoietin production.17 Additional contributory effects of these proinflammatory cytokines include induction of a state of relative iron deficiency, vitamin deficiency, and altered iron metabolism in the bone marrow.6,18 Anemia, therefore, is a result of both a blunted response to erythropoietin and abnormalities in iron metabolism.
 Laboratory Evaluation of Anemia in the Intensive Care Unit
Laboratory Evaluation of Anemia in the Intensive Care Unit
Iron absorbed from food or released from stores circulates in plasma bound to transferrin, the iron transport protein. This iron-transferrin complex interacts with a specific transferrin receptor protein on the surface of early erythroid cells. This complex is then internalized and the iron released intracellularly. Within the erythroid cells, iron in excess of that needed for Hb synthesis binds to the storage protein, apoferritin, forming ferritin. Iron in the ferritin pool can be released and reused in the iron metabolism pathway. The levels of ferritin in serum correlate with total body iron stores and are therefore a suitable laboratory estimate of iron stores.19 During maturation of reticulocytes to erythrocytes, the cells lose all activities of the Hb-synthesizing system, including surface expression of the transferrin receptors, which are released into the circulation.20 Levels of transferrin receptor protein in the circulation provide a quantitative measure of total erythropoiesis and can be used to measure the expansion of the erythroid marrow in response to recombinant erythropoietin therapy. Serum iron levels represent the amount of circulating iron bound to transferrin. The total iron-binding capacity is an indirect measure of the circulating transferrin concentration.
 Management
Management
Red Cell Transfusion
Transfusion of PRBCs remains the standard approach for the management of anemia in critically ill patients. Most transfusions are administered in response to a particular Hb level, the “transfusion trigger.” Historically, transfusion was indicated for Hb concentrations below 10 g/dL. However, several considerations suggest a need to critically reevaluate this approach. First, scientific evidence suggests that most critically ill patients can safely tolerate lower Hb levels. Second, PRBC transfusions are associated with numerous potential complications. Third, blood is a scarce and costly resource that may not always be available,21 hence its use must be limited to those most likely to benefit. Finally, transfusions are associated with worse clinical outcomes. Transfusion of PRBCs must therefore be used for a physiologic indication and not in response to a transfusion trigger. The goals of these transfusions are to treat hemorrhage not responsive to fluid resuscitation and to correct hypoperfusion (as evidenced by blood lactate concentrations or base deficit measurements) not responsive to fluid resuscitation.
In recent years, evidence has begun to accumulate against the traditional liberal strategy of transfusion to achieve Hb concentration ≥10 g/dL. In the ABC trial, a prospective observational study of 3534 patients from 146 western European ICUs, 37% of all patients were transfused while in the ICU. The majority of transfusions were administered during the first week of ICU stay. Transfusion was more common in the elderly and in those with ICU stays longer than 1 week. Mortality, both in the ICU and overall, was significantly higher in the transfused group than for the group which avoided transfusion (18.5% versus 10.1%, P<0.001 for ICU death and 29.0% versus 14.9%, P<0.001 for overall mortality). The differences persisted even after the patients were matched for the degree of organ dysfunction.5 In addition, transfused patients had longer lengths of stay and more severe degrees of organ failure. The CRIT study was a prospective, multicenter, observational study of 284 ICUs in 213 hospitals in the United States. Overall, 44% of patients were transfused, most often within the first week of ICU admission; transfusion was independently associated with longer ICU and hospital stays and increased mortality.6 Walsh and colleagues prospectively collected data on 1023 sequential admissions in 10 ICUs over 100 days in Scotland. Approximately 40% of patients were transfused, even with the application of evidence-based transfusion guidelines.22 The multicenter trials group of the American Burn Association studied patients with ≥20% total body surface burns at 21 burn centers in the United States and Canada. Overall, they found that nearly 75% of patients were transfused during their hospital stay, receiving a mean of 14 units. The number of units transfused correlated significantly with the number of infections and mortality.23 In a prospective observational study by the North Thames Blood Interest Group, 53% of patients were transfused for a mean pretransfusion Hb level of 8.5 g/dL. About two-thirds were transfused for low Hb levels and only 25% for hemorrhage. ICU mortality in the transfused patients was significantly higher than in the nontransfused patients (24.8% versus 17.7%, respectively).24
There is increasing recognition that anemia is well tolerated in critically ill patients. Much of clinical evidence in support of this approach comes from studies in Jehovah’s Witness patients, who refuse to accept PRBC transfusions on religious grounds. Mortality increases significantly at Hb values below 5 g/dL, more so in individuals older than 50 years of age.25 In conscious health volunteers, isovolemic dilution was performed to reduce the Hb concentration from 13.1 g/dL to 5 g/dL. Critical oxygen delivery was assessed by oxygen consumption, blood lactate concentration, and changes in the ST segment on the electrocardiogram. Oxygen consumption increased, but no increase in lactate concentration was found, suggesting that resting healthy humans can tolerate acute reductions in Hb to levels of 5 g/dL without the development of inadequate tissue perfusion.26
Clearly the risks of anemia must be balanced against the potentially deleterious effects of transfusion, especially since the efficacy of PRBC transfusions to augment oxygen delivery and the impact of this increase on tissue metabolism and clinical outcome remain unproven. In a recent meta-analysis, Marik and Corwin performed a systematic review of the literature and analyzed outcomes in 272,596 patients as reported in 45 studies. Blood transfusion was associated with an increased risk of death (pooled odds ratio 1.69, 95% confidence interval [CI] 1.46–1.92), increased risk of infectious complications (pooled odds ratio 2.5, 95% CI 1.52–2.44), and an increased risk of the development of acute respiratory distress syndrome (ARDS) (pooled odds ratio 1.88, 95% CI 1.66–3.34).27
The only absolute indication for PRBC transfusion is in the therapy of hemorrhagic shock.28 However, only 20% of transfusions are used for this indication.
Most transfusions in the ICU are administered for the treatment of anemia. In the CRIT trial, over 90% of transfusions were given for this reason.6 Perceived benefits of transfusion include increase in oxygen delivery to the tissues; increase in the cell mass and blood volume; alleviation of symptoms of anemia, including dyspnea, fatigue, and diminished exercise tolerance; and relief of cardiac effects. The optimal Hb concentration remains unknown and is likely influenced by the premorbid health status, disease process, and other unknown factors. Based on studies involving acute isovolemic reductions of blood Hb concentration, it has been demonstrated that reduction of the Hb concentration to levels of 5 g/dL does not produce evidence of inadequate systemic critical oxygen delivery as evidenced by blood lactate concentration26; significant cognitive changes were noted, however.29 These effects were not seen when isovolemic dilution was performed to Hb levels of 7 g/dL. Clinical evidence of the validity of these findings is seen in the seminal Transfusion Requirements in Critical Care (TRICC) trial and has been instrumental in changing transfusion practices over the last decade.30 In this study, 838 euvolemic critically ill patients with Hb levels less than 9 g/dL were enrolled. Of these, 418 patients were randomly assigned to a restrictive transfusion strategy, where transfusion was provided if the Hb level fell below 7 g/dL, with a goal of maintaining circulating Hb concentration between 7 and 9 g/dL; and 420 patients were assigned to the liberal transfusion group and received transfusions for Hb levels of less than 10 g/dL, with transfusions provided to keep the Hb between 10 and 12 g/dL. Overall the 30-day mortality was similar between the two groups (18.7% versus 23.3%, P=0.11). However, a significantly lower mortality was seen with a restrictive transfusion strategy in those less severely ill who had APACHE II scores of ≤20 (8.7% versus 16.1%, P=0.03) and in those younger than 55 years of age (5.7% versus 13.0%, P=0.02). No difference in mortality was observed in those with stable, clinically significant cardiac disease (20.5% versus 22.9%, P=0.69). This strategy resulted in a 54% decrease in average number of units transfused and avoidance of transfusion in 33% of patients. Lowering of the transfusion threshold, therefore, is a simple and inexpensive strategy for improving outcome for critically ill patients. Caution must be used in applying this restrictive transfusion strategy to those patients with acute myocardial ischemia and unstable angina, as this group was excluded from the TRICC trial. Compensatory cardiac mechanisms in anemic patients include increases in blood flow during rest and a redistribution of blood away from the endocardium. In the presence of significant coronary artery disease, these adaptive changes are poorly tolerated, and anemic patients with myocardial infarction may have increased mortality.31
Adverse Effects of Transfusion
A large proportion of ICU patients continue to receive PRBC transfusions for anemia, exposing them to serious risks, including transmission of infectious diseases, immune-mediated reactions (acute or delayed hemolytic reactions, febrile allergic reactions, anaphylaxis, and graft-versus-host disease), and non–immune related complications (fluid overload, hypothermia, electrolyte toxicity, and iron overload). Transfusion-related complications are encountered in approximately 4% of PRBC transfusions.6 The risk of adverse outcomes increases incrementally with each unit of PRBCs transfused.32,33 In an observational cohort study of 5814 patients undergoing coronary artery bypass grafting, each unit of PRBC transfused resulted in more than 100% odds of renal dysfunction, 79% odds for the need for mechanical ventilation for over 72 hours, 76% increase in odds for developing a serious postoperative infection, a 55% increase in odds for postoperative cardiac morbidity, and a 37% increase in odds for postoperative neurologic morbidity. Overall, there was a 73% increase in the odds of a major morbidity for each unit transfused (Table 19-2).32
TABLE 19-2 Potential Adverse Consequences Associated with Red Cell Transfusion90
| Infectious Complications | |
| Human immunodeficiency virus infection Human T-lymphotropic virus infection Hepatitis C virus infection Hepatitis B virus infection Parvovirus B19 virus infection Bacterial infections (Staphylococcus, streptococci, Yersinia enterocolitica, etc.) Parasitic infections (Chagas disease) | 1 in 2.3 million 1 in 2 million 1 in 1.8 million 1 in 350,000 1 in 10,000 1 in 250,000 1 in 29,000 donors seropositive |
| Noninfectious Complications | |
| Hemolytic transfusion reactions Delayed hemolytic transfusion reaction Febrile nonhemolytic transfusion reactions Major allergic reactions ABO mismatching Transfusion-related acute lung injury (TRALI) Transfusion-related immunomodulation (TRIM) Transfusion-associated circulatory overload (TACO) Coagulopathy Iron overload Hypothermia Hyperkalemia Thrombocytopenia Pulmonary hypertension | 1 in 10,000 to 1 in 50,000 1 in 1500 1 in 100 to 35 in 100 1 in 20,000 to 1 in 50,000 1 in 14,000 to 1 in 38,000 1 in 5000 1 in 100 Observed once 2 blood volumes replaced Observed after transfusion of 10 to 15 units |
With advances in screening and improvements in blood banking technology, transmission of infectious agents is less common. Current estimates of the risk of infection per unit of blood are approximately 1 in 2 million for human immunodeficiency virus (HIV), 1 in 1 million for hepatitis C virus, and 1 in 100,000 for hepatitis B virus.34 The most common transfusion-related infections are secondary to bacterial contamination, which has an incidence of 12.6 events per 1 million units of allogeneic blood components transfused.35 The risk of bacterial contamination is higher for PRBCs than for whole blood. Transfusion-related bacterial infections are most often caused by gram-positive organisms (e.g., staphylococcal spp., streptococcal spp., 58%) but also may be caused by gram-negative organisms (e.g., Yersinia enterocolitica, 32%). About 10% of these infections will result in a fatal outcome.35 Increasing global travel has led to the emergence of infectious diseases not usually seen in the United States. Chagas disease, caused by Trypanosoma cruzi, is endemic in much of South and Central America. Immigrants from these endemic areas now form an increasing proportion of the blood donor pool. This issue is especially relevant in regions with high immigrant populations. In two such cities, Los Angeles and Miami, seropositive rates among donors were 1 in 7500 and 1 in 9000 and have been increasing.36 Once acquired, the parasitemia persists long after acquisition of the infection.37
Major ABO mismatching is estimated to occur in 1 of 138,673 PRBC units transfused and results in 1 death per 2 million units transfused.35 Incompatibility also may result from antigens not routinely detected by current antibody assays. As a consequence, fatal acute hemolytic reactions still occur in 1 of every 250,000 to 1 million transfusions, and 1 patient per 1000 demonstrates the clinical manifestations of a delayed hemolytic transfusion reaction.38
Transfusion-related acute lung injury (TRALI) is a potentially serious pulmonary complication of transfusion. In severe cases, its clinical presentation is similar to that of the acute respiratory distress syndrome (ARDS).39 Although initially described by Bernard in 195140 as noncardiogenic pulmonary edema related to transfusion, the term TRALI was coined by Papovsky et al.41 TRALI presents with dyspnea and bilateral pulmonary edema during or within up to 6 hours of a transfusion, with no other risk factors to explain its development. It must be distinguished from pulmonary insufficiency due to circulatory overload, where the central venous pressure and pulmonary artery wedge pressure would be elevated. Hypoxemia, fever, hypotension, tachycardia, and cyanosis also may occur. Most often, symptoms appear within 1 or 2 hours following transfusion, but a delayed form with dyspnea appearing as late as 48 hours after transfusion has been reported. The chest x-ray shows bilateral infiltrates, which may progress and cause whiteout of the entire lung field. The criteria for clinical diagnosis of TRALI42 include severe hypoxemia (with PaO2/FIO2 <300 or O2 saturation <90%), acute respiratory distress within 6 hours of a transfusion in the absence of evidence of circulatory overload, and x-ray evidence of bilateral pulmonary infiltrates. Differential diagnosis includes transfusion-associated circulatory overload, cardiac diseases, allergic and anaphylactic transfusion reactions, and bacterial contamination of the blood. Although the exact incidence is unknown, TRALI is estimated to occur in 1 of every 5000 transfusions43 and has a mortality rate of 5% to 10%. Current evidence suggests two forms of TRALI: immune and nonimmune. Potential mediators include antileukocytic antibodies, products of lipid peroxidation, and other as yet unrecognized agents. The neutrophil is the key effector cell. Transfusions from multiparous female donors, owing to exposure to paternal leukocytes, are associated with the highest risk for the development of TRALI in the recipient.44 Treatment is currently limited to supportive measures.
Transfusion-related immunomodulation (TRIM) results in an increased incidence of bacterial infections, cancer recurrence, and organ dysfunction.45,46 Opelz and colleagues first suggested clinical evidence of transfusion-associated immunomodulation in 1973, when improved renal allograft survival was observed in patients transfused prior to transplantation.47 Current evidence implicates transfusions in the development of nosocomial infections including wound infections, pneumonia, and sepsis. In a prospective observational study, Taylor et al. found a significant association between transfusion and development of nosocomial infections (14.3% versus 5.3%, P<0.0001). In addition, mortality and length of stay were increased in the transfused group. The risk of infection increases 9.7% for each unit of PRBC transfused.48 Development of these infectious complications results not only in increased length of stay but in increased in-hospital deaths and increased costs as well.49 These effects may be reduced by the use of prestorage leukocyte depletion.50
Other complications include transfusion-associated circulatory overload with the development of fluid overload and pulmonary edema, multisystem organ failure, systemic inflammatory response syndrome,51,52 hypothermia, coagulopathy, thrombocytopenia, hyperkalemia, and pulmonary hypertension with an increase in pulmonary vascular resistance and decreased right ventricular ejection fraction.53
Finally, the transfusion of PRBCs may not augment the oxygen-carrying capacity of blood. This results from development of the “storage lesion” due to changes in red blood cells that occur during ex vivo storage. These changes are both structural and functional54,55 and include reduced deformability impeding microvascular flow,56 altered adhesiveness and aggregation,57 reduced intracellular levels of 2,3-diphosphoglycerate (2,3-DPG, which shifts the oxyhemoglobin dissociation curve to the left and reduces oxygen delivery to the tissues), reduction in levels of nitric oxide and adenosine triphosphate,58 and accumulation of bioactive compounds with proinflammatory activity.59 The risk of complications increases with the duration of storage.60,61 Although the U.S. Food and Drug Administration (FDA) approves storage of red cells for up to 42 days, transfusion of blood older than 2 weeks appears to be associated with a significantly worse outcome. Koch and colleagues examined data from 6002 patients undergoing coronary artery bypass grafting, heart valve surgery, or both. “Newer blood” stored for less than 14 days was administered to 2872 patients, while the remaining 3130 received “older blood” stored for ≥14 days. Patients given older blood had higher rates of in-hospital mortality (2.8% versus 1.7%, P=0.004), need for longer duration of intubation (9.7% versus 5.6%, P<0.001), higher incidence of acute renal failure (2.7% versus 1.6%, P=0.003), and higher incidence of sepsis (4.0% versus 2.8%, P=0.001). The difference in mortality persisted even at 1 year after transfusion (7.4% versus 11.0%; P<0.001).62

Full access? Get Clinical Tree


