Anatomy and Physiology of Head Pain
Karl Messlinger
Jonathan O. Dostrovsky
Andrew M. Strassman
A large body of indirect evidence supports the idea that some types of headaches, including migraine, are caused by activity in nociceptive afferents that innervate the cranial meninges, particularly the dura mater encephali and large intracerebral blood vessels (133,146). This idea was initially based on the classical intraoperative experiments by Ray and Wolff (149), followed by supplementary studies of other investigators, who demonstrated that pain, but not other sensations, can be evoked by electrical, mechanical, thermal, or chemical stimulation of dural blood vessels and sinuses or large intracerebral arteries (26). Importantly, the painful sensations were referred to the trigeminal dermatomes where typically headaches are localized (144). These early studies formed the basis of many anatomic and physiologic examinations that were made with regard to the pathophysiology of headaches.
MENINGEAL REPRESENTATION IN THE TRIGEMINAL GANGLION
Cranial nerve V, arising from the trigeminal ganglion, also referred to as the semilunar or Gasserian ganglion, conveys sensory information from the orofacial region, including intracranial structures, to the central nervous system. Mayberg et al. (123) and Steiger and Meakin (163) traced meningeal afferents by applying horseradish peroxidase (HRP) to pial and dural structures in the cat. Afferents around the medial meningeal artery projected predominantly to the ophthalmic division (V1) of the ipsilateral trigeminal ganglion, but to a minor extent also to the maxillary (V2) and mandibular (V3) divisions (123). The basal dura mater in the middle cranial fossa was represented mainly in V3 (163). The finding that all three divisions of the trigeminal nerve, although not equally, contribute to the innervation of the meninges is in accordance with old anatomic observations in primates (126). Retrograde labeling of nerve fibers around basal intracranial arteries, from which head pain can be provoked in humans (149), was found not only in the trigeminal ganglion but also in the first and second spinal ganglia in the rat (9). Because of the limited experimental access to intracerebral arteries, most of the morphologic and nearly all functional studies have focused on the innervation of the cranial dura mater and dural venous sinuses.
INNERVATION OF THE CRANIAL DURA MATER AND INTRACEREBRAL BLOOD VESSELS
The dura mater encephali is richly innervated by afferent nerve fibers, most of which originate in the ipsilateral trigeminal ganglion, and by sympathetic fibers predominantly arising from the ipsilateral superior cervical ganglion (52,100,178). In addition, a comparatively sparse parasympathetic innervation was described (5,52). The innervation of intracerebral (pial) blood vessels is similarly organized (67,173), but with more parasympathetic fibers that originate mainly from the internal carotid and sphenopalatine ganglia (172). Several studies described neuropeptide immunoreactive nerve fibers in the dura mater (99,183) and around cerebral (pial) blood vessels in different species including man (50,117,177). Meningeal nerve fibers immunoreactive for substance P (SP), neurokinin A, and calcitonin gene-related peptide (CGRP) are thought to belong to the afferent (sensory) system, and nerve fibers immunopositive for neuropeptide Y are most likely of sympathetic and those immunoreactive for vasoactive intestinal polypeptide of parasympathetic origin. Light and electron microscopic studies of the rat dura mater have shown that the peptidergic (sensory) nerve fibers form a dense network both around blood vessels as well as in nonvascular regions (99,132,171) (Fig. 10-1).
CGRP-immunoreactive nerve fibers and trigeminal ganglion cells innervating the meninges are much more abundant than SP-immunoreactive afferents (178), suggesting an enrichment of CGRP in trigeminal fibers that supply
intracranial vessels (140). Colocalization of SP and CGRP was shown in a minor proportion of trigeminal ganglion cells (110). Subarachnoid hemorrhage in the rat substantially reduced the SP-immunoreactive nerve fibers of the dura mater, but left the CGRP-immunoreactive innervation unchanged (101). Taken together, the CGRP-containing trigeminal innervation of intracranial structures seems to play an important role in meningeal nociception (see Chapters 16 and 33).
intracranial vessels (140). Colocalization of SP and CGRP was shown in a minor proportion of trigeminal ganglion cells (110). Subarachnoid hemorrhage in the rat substantially reduced the SP-immunoreactive nerve fibers of the dura mater, but left the CGRP-immunoreactive innervation unchanged (101). Taken together, the CGRP-containing trigeminal innervation of intracranial structures seems to play an important role in meningeal nociception (see Chapters 16 and 33).
Meningeal sensory fibers can be stimulated to release neuropeptides from their peripheral endings in the meninges, where they can evoke components of neurogenic inflammation, including dural plasma extravasation, as well as dural and pial vasodilation (51,107,121,124). SP acting at the neurokinin-1 receptor appears to be responsible for extravasation, whereas CGRP mediates the neurogenic vasodilation (24,107,156). Neurogenic inflammation has been hypothesized to play a role in headache pathogenesis (see Chapter 33).
SENSORY RESPONSES OF DURAL PRIMARY AFFERENT NEURONS
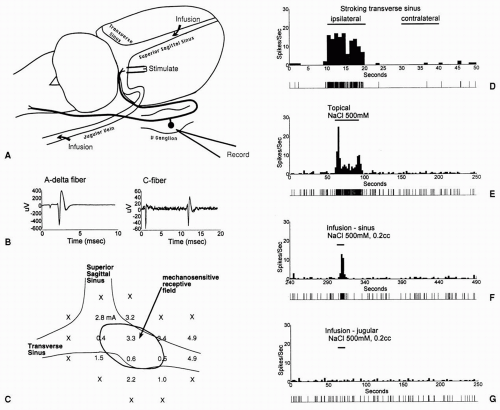
Activation of meningeal sensory fibers is thought to be involved in some types of headaches, including migraine. To identify the types of stimuli that are capable of activating meningeal sensory fibers, electrophysiologic recording studies in animals have examined the response properties of primary afferent neurons that innervate the cranial dura. In these studies, single-unit recordings were obtained from the trigeminal ganglion (46,169) or the nasociliary nerve (18). Dural afferents were typically identified by their responses to electrical stimulation of dural sites on or around the dural venous sinuses, namely, superior sagittal or transverse sinus, or the middle meningeal artery (MMA). Based on their response latencies, the majority of the neurons could be classified as C or Aδ, although a substantial number of Aβ fibers was also present (113), which is in agreement with anatomic observations (171).
Many of the neurons exhibit mechanosensitive receptive fields on the dura, from which they can be activated by punctate probing, stroking, or traction. Some neurons also respond to thermal stimuli, in either the warming or cooling direction (18,46). In addition, neurons were excited by chemical stimuli such as hypertonic saline, applied either topically to the dura or by intravascular infusion into the superior sagittal sinus (SSS) (169) (Fig. 10-2). Neurons could also be activated by dural application of a number of algesic agents, including potassium chloride, capsaicin, buffer solutions of low or high osmolarity, or a mixture of inflammatory mediators (bradykinin, PGE2, serotonin, histamine). Besides activating the neurons, the inflammatory mediators produced a sensitization, or enhancement, of responses to mechanical stimuli. Such sensitization of meningeal primary afferents could contribute to clinical symptoms of headache (see Chapter 12). The polymodal response properties of the dural afferent neurons, and their excitatory and sensitizing responses to algesic and inflammatory agents, support the idea that some of these neurons serve a nociceptive function, and that they might be activated under pathologic conditions such as increased intracranial pressure or meningitis (18,112).
ORGANIZATION OF THE TRIGEMINAL NUCLEUS
The central processes of trigeminal primary afferents forming the sensory root of the trigeminal nerve enter the brain stem at the pontine level and terminate in
sensory nuclei, which as a whole are called the trigeminal brain stem nuclear complex (TBNC). The TBNC is composed of the principal sensory nucleus (Vp) and the spinal trigeminal nucleus (Vsp) (Fig. 10-3). The large-diameter, non-nociceptive afferents of the trigeminal nerve terminate in the Vp, whereas both the large diameter and small diameter fibers descend in the spinal trigeminal tract (SVT). The descending axons give rise to collaterals that extend into the Vsp, which is subdivided into three subnuclei (141) (see Fig. 10-3): a rostral subnucleus oralis (Vo), a middle subnucleus interpolaris (Vi), and a caudal subnucleus caudalis (Vc). The Vc is often referred to as the medullary dorsal horn (MDH) because of its anatomic and physiologic similarities to the spinal dorsal horn. Olszewski (141) identified three histologically different regions in the MDH: an outer marginal region, the substantia
gelatinosa, and a deep magnocellular region. Later Gobel et al. (60) proposed a laminar subdivision of the MDH similar to Rexed’s nomenclature of the spinal dorsal horn (151) in which lamina I corresponds to the marginal layer, lamina II to the substantia gelatinosa, and laminae III and IV to the magnocellular region. The most ventral lamina V merges with the medullary reticular formation (138) so that the ventral boundary of the MDH is not clearly defined. Adjacent to the rostral Vsp, groups of neurons are found intermingled in the spinal trigeminal tract down to the transition between Vi and Vc (see Fig. 10-3). These cells are referred to as the interstitial
islands of Cajal, or as the paratrigeminal or interstitial nucleus (145). The interstitial nucleus cells have been suggested to be functional homologs to neurons in laminae I and II of the Vc, because both tend to be nociceptive specific and have restricted receptive fields (44,71, 159).
sensory nuclei, which as a whole are called the trigeminal brain stem nuclear complex (TBNC). The TBNC is composed of the principal sensory nucleus (Vp) and the spinal trigeminal nucleus (Vsp) (Fig. 10-3). The large-diameter, non-nociceptive afferents of the trigeminal nerve terminate in the Vp, whereas both the large diameter and small diameter fibers descend in the spinal trigeminal tract (SVT). The descending axons give rise to collaterals that extend into the Vsp, which is subdivided into three subnuclei (141) (see Fig. 10-3): a rostral subnucleus oralis (Vo), a middle subnucleus interpolaris (Vi), and a caudal subnucleus caudalis (Vc). The Vc is often referred to as the medullary dorsal horn (MDH) because of its anatomic and physiologic similarities to the spinal dorsal horn. Olszewski (141) identified three histologically different regions in the MDH: an outer marginal region, the substantia
gelatinosa, and a deep magnocellular region. Later Gobel et al. (60) proposed a laminar subdivision of the MDH similar to Rexed’s nomenclature of the spinal dorsal horn (151) in which lamina I corresponds to the marginal layer, lamina II to the substantia gelatinosa, and laminae III and IV to the magnocellular region. The most ventral lamina V merges with the medullary reticular formation (138) so that the ventral boundary of the MDH is not clearly defined. Adjacent to the rostral Vsp, groups of neurons are found intermingled in the spinal trigeminal tract down to the transition between Vi and Vc (see Fig. 10-3). These cells are referred to as the interstitial
islands of Cajal, or as the paratrigeminal or interstitial nucleus (145). The interstitial nucleus cells have been suggested to be functional homologs to neurons in laminae I and II of the Vc, because both tend to be nociceptive specific and have restricted receptive fields (44,71, 159).
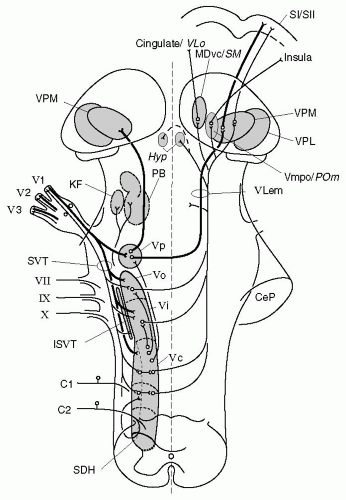 FIGURE 10-3. Schematic representation of ascending pathways of the central trigeminal system with priority to nociception. The figure is based on a representation of the human trigeminal system by Nieuwenhuys et al. (137), but includes trigeminal structures found in other species, which may differ in part from the trigeminal system of primates. Contours of nuclei are simplified; symbols for single cell bodies and nerve fibers represent populations of neurons. The TBNC includes subnucleus principalis (Vp) and the spinal trigeminal nucleus, which consists of subnuclei oralis (Vo), interpolaris (Vi), and caudalis (Vc). Primary afferents from the trigeminal divisions (V1-3) project to all subnuclei of the TBNC, thick myelinated fibers mainly to Vp, and thin A and C fibers preferentially to caudal subnuclei and the first spinal segments (C1-2). Minor projections to the spinal trigeminal nucleus run through nerves VII, IX, and X (not further described). Intersubnuclear connections are shown originating from Vc but may connect all subnuclei of the TBNC. Neurons from Vp ascend to the ipsilateral and to the contralateral thalamus, forming the trigeminal lemniscus (VLem). Trigeminothalamic neurons involved in nociception project via a crossed pathway mainly to the VMpo (in primates), the ventroposteromedial nucleus (VPM), the medial region of the POm, and the ventrocaudal medial dorsal nucleus (MDvc; in primates) or nucleus submedius (SM; in rat and cat) in the medial thalamus. There is also a significant projection from the TBNC to the hypothalamus (Hyp) and to the pontine parabrachial (PB) and Kölliker-Fuse (KF) nuclei. Abbreviations: CeP, cerebellar pedunculus; SDH, spinal dorsal horn; SI, primary somatosensory cortex; SVT, spinal trigeminal tract; ISVT, interstitial nucleus of the SVT; VPL, ventroposterolateral thalamic nucleus. The main cortical projections from these thalamic nuclei are to the cingulate cortex (in primate) or ventrolateral orbital cortex (VLo, in cat and rat), the insula, and the primary and secondary somatosensory cortices (SI and SII). |
Numerous anatomic and electrophysiologic studies (70,158,170) have demonstrated that the Vp, the spinal trigeminal tract, and the subnuclei of the Vsp are topographically organized in a largely ventrodorsal direction. Mandibular afferents terminate preferentially in the dorsal aspect of each trigeminal subnucleus (dorsomedial in the MDH), ophthalmic afferents terminate ventrally (ventrolateral in MDH), and maxillary terminals are interposed. The rostrocaudal organization of the TBNC is less clear, but within the Vc, the rostrocaudal axis of the face is represented from rostral to caudal (193). The results of early anatomic (176) and neurophysiologic (105,176) studies suggest that each subnucleus receives information from all parts of the head. Jacquin et al. (90) labeled various mandibular nerves in the rat with HRP and found that they projected to all trigeminal subnuclei, although the anterior oral afferents tended to terminate most heavily in the rostral TBNC, whereas the posterior perioral-auricular afferents terminated preferentially in the caudal aspect of the complex. It is not clear if a similar somatotopic distribution in ventrodorsal and rostrocaudal directions exists for intracranial trigeminal structures.
On the basis of clinical observations and animal studies, it has long been recognized that the Vc is primarily responsible for processing nociceptive and temperature information from the face and head, whereas the Vp is involved in processing tactile information. Isolated lesions of the Vc caused ipsilaterally complete or partial loss of pain and temperature sensation, whereas tactile sensations remained nearly intact (116). This clinical experience led Sjoqvist (161) to develop the method of trigeminal tractotomy for the relief of facial pain, in which the spinal trigeminal tract at the level of the obex was transected. The clinical data were supplemented with a large body of neurophysiologic evidence demonstrating the necessity of the Vc in the perception of pain in trigeminal tissues.
There is evidence also for a role of the rostral portions of the trigeminal nuclear complex in nociception, which initially came from observations that the loss of facial pain sensation produced by obex tractotomy was not complete, in most cases at least partially sparing the intraoral and perioral regions (106,196). Similarly, reflex or behavioral responsiveness to noxious orofacial stimuli may persist following tractotomy or Vc lesions in animals (20,184); conversely, nociceptive responsiveness may be diminished by more rostral lesions of the trigeminal complex (20,61). There is also evidence for termination of nociceptive afferents and nociceptive responsive neurons in Vi and Vo.
TERMINATIONS OF TRIGEMINAL AFFERENTS IN SPINAL TRIGEMINAL SUBNUCLEI
The distribution of nociceptive afferent terminals in the trigeminal brain stem has been studied by axonal tracing combined with immunohistochemistry. Hayashi (69) and Jacquin et al. (92,93) injected HRP into functionally identified facial primary afferents in the Vsp of the cat and the rat, respectively, to examine the central terminations of these labeled axons. They found high-threshold mechanoreceptive (nociceptive) Aδ afferents forming extensive terminal arbors in the superficial Vi and, most pronounced, in lamina I and, to a lesser extent, outer lamina II of the Vc (69). In the rat, a second termination area was localized in laminae III to V of the Vc (92). In line with these findings, the sensory projection from the cornea, which is thought to be mainly nociceptive, was shown to be focused in the outer laminae of the Vc (142). Apart from the heavy projection of nociceptive primary afferents to the Vc, a sparse projection of corneal and tooth pulp afferents was shown also in Vp and Vo (142,185). Corresponding to the distribution of nociceptive afferent terminals in the Vsp visualized by the HRP technique, SP and CGRP immunoreactive nerve fibers have been demonstrated in different species preferentially around the substantia gelatinosa of the Vc and the transition zone between Vi and Vc (Vi/Vc) (17,143,174). In the ferret, SP and CGRP immunoreactivity was most dense in outer laminae of the Vc and in the caudal part of the Vi (4), less in Vo and Vp. Trigeminal rhizotomy in the cat caused disappearance of most of the CGRP immunoreactive fibers throughout the TBNC, whereas a considerable number of SP immunoreactive fibers remained intact (73,174), suggesting that these are of central origin. Electron microscopic immunocytochemistry in the cat Vsp revealed CGRP immunoreactivity within the substantia gelatinosa in axon terminals, which were presynaptic to dendritic profiles and postsynaptic to other fibers (74). SP in particular has been implicated in the nociceptive processing within the Vsp. Stimulation of the rat dura mater with acidic solution provoked release of immunoreactive SP in the medullary trigeminal brain stem measured with the microprobe technique (152). Henry et al. (75) found that iontophoretic administration of SP in the cat Vc selectively activates nociceptive neurons. CGRP and SP receptor expression of trigeminal fibers in the human Vc was found by immunohistochemistry (162).
CONNECTIONS BETWEEN TRIGEMINAL SUBNUCLEI
The nociceptive information processed in the subnuclei of the TBNC does not simply reflect the primary afferent input. Rather there are connections between the various
subnuclei that allow information transfer within the trigeminal system (see Figure 10-3). Anatomic studies using degeneration techniques have shown projections from Vc to more rostral nuclei of the TBNC (164), which was confirmed by physiologic experiments showing antidromic activation of Vc neurons from Vo (84). Jacquin et al. (91) used anterograde tracing to demonstrate ascending connections between each of the TBNC subnuclei in the rat, as well as a weak projection to the contralateral Vsp. It appears likely, therefore, that the MDH transmits information to all rostral components of the TBNC.
subnuclei that allow information transfer within the trigeminal system (see Figure 10-3). Anatomic studies using degeneration techniques have shown projections from Vc to more rostral nuclei of the TBNC (164), which was confirmed by physiologic experiments showing antidromic activation of Vc neurons from Vo (84). Jacquin et al. (91) used anterograde tracing to demonstrate ascending connections between each of the TBNC subnuclei in the rat, as well as a weak projection to the contralateral Vsp. It appears likely, therefore, that the MDH transmits information to all rostral components of the TBNC.
Because Vc is the main if not the only subnucleus that receives a direct projection from unmyelinated trigeminal primary afferents, a number of studies have examined whether Vc is responsible for relaying nociceptive inputs to the more rostral subnuclei. Hallas and Jacquin (66) reported that the number of Vi neurons responsive to noxious facial stimuli does not change following surgical interruption of the ascending projection from the Vc. However, Greenwood and Sessle (62) showed that the responses of neurons in Vo and Vp to noxious stimuli were reversibly depressed by cold block of the ascending input from the Vc. Similarly, Davis and Dostrovsky (42) found that cold block of Vc abolished the responses to cerebrovascular stimulation of some, but not all neurons in Vi and Vo. These studies indicate that nociceptive inputs reach the rostral subnuclei in part via a relay in the Vc. This conclusion is further supported by the recent finding that C fiber-evoked responses of Vo neurons can be suppressed by microinjection of morphine into the Vc, but not by microinjection into the Vo (38).
RESPONSE PROPERTIES OF NEURONS IN THE TRIGEMINAL NUCLEUS
The majority of neurons that have been recorded in the Vc exhibit orofacial receptive fields from which they can be activated by appropriate mechanical or thermal stimulation. The receptive fields of Vc neurons are organized in a somatotopic representation of the trigeminal dermatome that is continuous with the representation of the posterior head and neck region in the upper cervical dorsal horn. The somatotopic organization in the Vc follows that of the primary afferent projections (see above), whereby the rostrocaudal axis of the face is represented from rostral to caudal in the Vc, and the dorsoventral axis of the face is represented from ventral to dorsal (or ventromedial to dorsolateral, depending on the species and the rostrocaudal level in the nucleus) (48,114).
As in the spinal dorsal horn, neurons in the Vc can be categorized as low-threshold mechanoreceptive (LTM), innocuous thermoreceptive, or nociceptive, according to their preferred modality of stimulation within their cutaneous or mucosal receptive field (82,83,135,147). The nociceptive neurons are further classified as nociceptive specific (NS) or wide dynamic range (WDR), based on their responses to graded intensities of mechanical stimulation. NS neurons respond solely to noxious stimulus intensities, whereas WDR neurons respond with increasing discharge frequency to increases in mechanical stimulus intensity from the innocuous (brushing) to the noxious (pinch) range (83,125,147,193). A distinct group of NS neurons has been described in the cat and monkey in lamina I of the spinal cord and Vc, termed heat, pinch, cold (HPC) (31,36). Both WDR and NS neurons receive primary afferent input from slowly conducting fibers (Aδ alone or Aδ plus C), whereas WDR neurons also receive input from Aβ fibers, consistent with their tactile sensitivity. The majority of WDR and NS neurons also respond to noxious heat applied to their cutaneous receptive field. A somewhat smaller proportion also respond to noxious cold stimuli (125).
Neurons in the Vc show a differential laminar distribution according to response class similar to that found in the spinal dorsal horn, with thermoreceptive and HPC neurons in the superficial laminae (I-II), LTM neurons in the deep laminae (III-IV and V), NS neurons preferentially in laminae I-II but also in V, and WDR neurons primarily in lamina V (4,31,34,83,86,147,193). Antidromically identified trigeminothalamic neurons are found mostly in laminae I-II and V and include both nociceptive (WDR, NS, and HPC) and thermoreceptive neurons (in lamina I only), but relatively few LTM neurons (36). LTM neuron input to the thalamus arises mainly from more rostral parts of the trigeminal complex (48) consistent with the relatively minor effect of Vc lesions on tactile sensation (161,191).
Nociceptive neurons in the Vc commonly receive inputs from noncutaneous tissues as well, including muscle (masseter and temporalis), temporomandibular joint, intranasal mucosa, cornea, tooth pulp, and blood vessels of the intracranial dura (4,19,46,136,155). The convergence of afferent input from separate superficial and deep peripheral tissues is also seen in nociceptive neurons in the spinal cord (16,189) and has been postulated as the basis for the clinical phenomenon of referred pain of deep or visceral origin. As has been found for spinal neurons, Vc neurons that receive inputs from deep tissues generally exhibit a cutaneous receptive field as well, and are usually nociceptive in their cutaneous response properties. The large, nociceptive cutaneous receptive fields exhibited by many neurons that receive convergent inputs from deep tissues are consistent with the diffuse, poorly localized, radiating quality that often characterizes pain of deep or visceral origin. Neurons expressing c-fos protein (a gene product that is induced following neuronal activation, particularly noxious stimulation) are found in greatest numbers in laminae I-II and, to a lesser extent, lamina V of Vc, and C1-2 following noxious stimulation of orofacial skin, cornea, tooth pulp, intraoral mucosa, or deep tissues such as dura or temporomandibular joint (68,81,89,128,167,170).

Full access? Get Clinical Tree