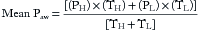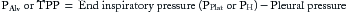Chapter 74
Alternative Modes of Ventilation in Acute Lung Injury 
Throughout this chapter the Bi-Level nomenclature refers to the mode of mechanical ventilation that intermittently cycles between two levels of continuous positive airway pressure (CPAP) and allows for spontaneous breaths throughout the entire respiratory cycle. Thus, Bi-Level ventilation is distinct from the biphasic intermittent positive airway pressure (BIPAP) mode used for non-invasive ventilation (Chapter 3). APRV refers to an “open lung” ventilatory strategy, which is a type of Bi-Level mode that places specific constraints on inflation and deflation pressure levels, cycle times, and pressure support (PS) levels during spontaneous breaths (Figure 74.1).
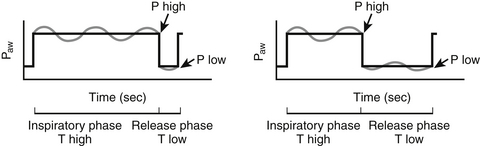
Figure 74.1 Pressure-time graphs of APRV versus standard Bi-Level.
APRV is hallmarked by longer inflation phases and brief release phases. Both allow spontaneous breathing (depicted by sinusoidal waves). Paw: airway pressure. (Adapted from Seymour CW, Frazer M, Reilly PM, et al: Airway pressure release and biphasic intermittent positive airway pressure ventilation: are they ready for prime time? J Trauma 62:1298-1308; discussion 1298-1308, 2007.)
Nomenclature and Description
With Bi-Level ventilation, the respiratory cycle commences at a high CPAP, designated as P high or PH, for a discrete time period, T high or TH. The amount of pressure and length of time of this inflation phase result in lung recruitment and oxygenation. Each inflation phase is coupled to a release phase with a set low pressure, P low or PL, and duration, T low or TL, thus completing a respiratory cycle (Figure 74.2). The release phase helps to eliminate CO2 and provide adequate ventilation. Importantly, integrated within and independent of each ventilatory cycle, the patient is allowed unrestricted spontaneous breaths via an active exhalation valve (AEV). While AEVs are not new, improved technology now allows the AEV in Bi-Level ventilation mode to open slightly for spontaneous respiration while maintaining constant airway pressure. These spontaneous efforts may or may not be assisted with pressure support (PS) (Chapter 2) and can augment oxygenation and ventilation. In essence, Bi-Level uses a high CPAP level to oxygenate with intermittent timed releases to a low CPAP level to achieve alveolar ventilation. Without spontaneous breathing by the patient, the pressure-time waveform of Bi-Level is similar to that of pressure control ventilation. To this end, Bi-Level should be considered a time triggered, pressure-limited, and time-cycled mode of mechanical ventilation. Synchronized versions of Bi-Level are now present that allow for patient triggering and cycling.
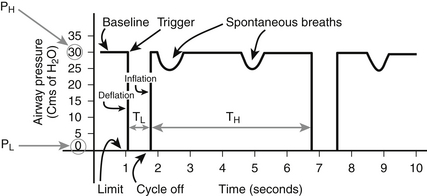
Figure 74.2 Pressure-time graph of APRV.
APRV is time triggered, pressure limited, and time cycled. (Adapted from Frawley PM, Habashi NM: Airway pressure release ventilation: theory and practice. AACN Clin Issues 12:234-246, 2001.)
APRV Concept, Indications, and Potential Advantages
In 1987, Stock et al first described and used APRV in patients with ALI. In the poorly compliant and fluid-filled lungs of ALI patients, the functional residual capacity (FRC) is reduced. In comparison to standard Bi-Level, APRV employs a longer TH to maximize the generated mean Paw (Equation 1) in an effort to restore FRC to a more favorable position on the pressure-volume curve (see Chapter 73, Figure 73.2). In addition, the timed-release phases in APRV are purposefully short in length and set to a PL of 0 cm H2O (see Figure 74.2). Thus, APRV employs high, but presumably safe, inflation pressures with very brief release (expiratory) phases during which expiratory flow continues until the ventilator starts the next inflation phase.
By creating an expiratory time that is very brief and not allowing the airway pressure to reach 0 cm H2O, a variable amount of intrinsic positive end-expiratory pressure (iPEEP) is generated (Chapter 2). This iPEEP helps to prevent those lung units recruited during previous inflation cycles from collapsing (de-recruiting) during subsequent release phases as well as decreasing the degree of repetitive opening and closing injury (atelectrauma). The desired iPEEP is attained by increasing or decreasing the expiratory (release) time. Decreasing the TL will increase the iPEEP and increasing the TL will decrease the iPEEP.
Disadvantages and Potential Limitations of APRV Use
In APRV, the negative pleural pressures generated by spontaneous breathing are real, variable, and distributed heterogeneously throughout the lung. For instance, in APRV with a set PH of 30 cm H2O, a generated pleural pressure of (−)10 cm H2O during a spontaneous patient effort would translate into a TPP of 40 cm H2O, a level considered unsafe in clinical practice. By limiting sedation and use in APRV, this concern may be exacerbated by allowing greater patient inspiratory effort and TPP swings. Furthermore, because APRV relies on a pressure mode of ventilation, VT (during the release phase) is dependent on lung compliance, pressure levels, airways resistance, and release time. Changes in any of these parameters can adversely lead to large unintended changes in delivered tidal volume. As will be described in greater detail, one of the greatest challenges when using APRV is setting an optimal duration of TL, which, in turn, depends on alveolar time constants (TCs). TC refers to the rate at which a lung unit empties (or fills) and is mathematically the product of airways resistance (Raw) and static compliance (CSt). Therefore, the diseased lung units at greatest risk for atelectrauma (e.g., lowest CSt) will have the shortest TC and unfortunately are the first to de-recruit during the release phase. Furthermore, the rapid release phases could impart shearing forces to lung epithelial and endothelial structures not previously recognized in more conventional modes of ventilation. ![]()
Clinical interest in APRV use has evolved from two basic premises: (1) APRV use as an alternative to standard modes of PC and VC for lung protective ventilation and (2) APRV use as a salvage therapy in ALI patients with refractory hypoxemia despite optimized settings on conventional mechanical ventilation. APRV may have several conceptual advantages in the ventilation of ALI patients (Box 74.E1). Despite chest radiographs often revealing homogeneous patterns of pulmonary infiltrates in ALI, the diseased lung is mechanically and histopathologically heterogeneous (Chapter 73). Regions of consolidated, atelectatic, aerated, and overinflated lung units not only exist next to one another, but their distribution throughout the lung varies considerably. In addition, gravitational forces typically contribute to dependent atelectasis across the sternovertebral axis in supine patients. Because of regional differences in lung mechanics in addition to regional differences in transpulmonary pressures, mechanically delivered tidal volumes are not uniformly distributed in ALI. Furthermore, patients with ALI often require deep sedation and sometimes pharmacologic paralysis, disrupting the normal contraction and movement of the diaphragm. When paralyzed and supine, the diaphragm is displaced in a manner such that the anterior portions of the lower lung zones receive preferential ventilation. Because perfusion continues to go preferentially to posterior segments (as a result of gravity), 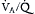 mismatch increases. By preserving spontaneous breathing in these patients, the actively contracting diaphragm may minimize the sternovertebral gradient for atelectasis by augmenting regional ventilation to the most dependent portions of the injured lungs. Furthermore, by generating negative pleural pressures in the spontaneously breathing patient, this regional increase in transpulmonary pressures leads to improved
mismatch increases. By preserving spontaneous breathing in these patients, the actively contracting diaphragm may minimize the sternovertebral gradient for atelectasis by augmenting regional ventilation to the most dependent portions of the injured lungs. Furthermore, by generating negative pleural pressures in the spontaneously breathing patient, this regional increase in transpulmonary pressures leads to improved 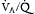 distribution and a reduced intrapulmonary shunt, particularly in the dependent portions of the injured lung. Therefore, central to the concept of APRV is the critical importance of preserving and encouraging spontaneous breathing by the patient throughout TH.
distribution and a reduced intrapulmonary shunt, particularly in the dependent portions of the injured lung. Therefore, central to the concept of APRV is the critical importance of preserving and encouraging spontaneous breathing by the patient throughout TH.