CHAPTER 12 Airway Management
Developmental anatomy*
The Upper Airway
Formation of the Cranial Vault and Base
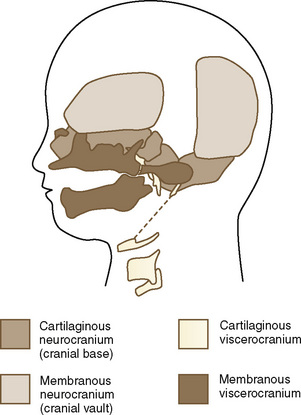
The skull is a critical factor in the development of the face and therefore the upper airway. The skull develops from a membranous and cartilaginous neurocranium (Fig. 12-1). The membranous neurocranium gives rise to the flat bones of the cranial vault, and the cartilaginous neurocranium (chondrocranium) forms the skull base. The flat bones of the neurocranium, which form sutures from edge to edge, also form fontanels where more than two bones meet. The base of the skull is formed from the cartilaginous neurocranium, which then becomes the base of the occipital bone, the sphenoid, the ethmoid and petrous bones, and portions of the temporal bone.
Craniovertebral Development
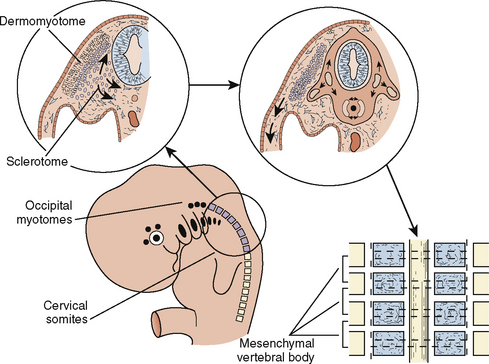
The paraxial mesoderm, a column of tissue on either side of the midline of the embryo, becomes divided into blocks of tissue (somites) at about the fourth week of development. Whereas most of the muscles of the head are derived from mesenchyme of the branchial arches, the cervical somites form the vertebrae of the neck that, under normal circumstances, undergo segmentation (Fig. 12-2). Failure of such segmentation can result in fusion and shortening with severely limited neck movement.
Clinical Correlation
Klippel-Feil syndrome is the result of varying combinations of fusions of the cervical vertebrae, such that the head appears to sit on the shoulders. The normal development of separate cervical vertebrae may be impaired, and fusion of adjacent vertebral bodies may occur. The degree of severity is variable; type I patients have a single-level fusion; type II patients have multiple, noncontiguous fused segments; and type III patients have multiple, contiguous fused segments (Samartzis et al., 2006). Klippel-Feil syndrome can occur with fetal alcohol syndrome, hemifacial microsomia (Goldenhar’s syndrome), and anomalies of the extremities. The neck is short, and the hairline is low. In addition, the neck can be webbed. There may be atlanto-occipital fusion. Laryngoscopy and intubation can be extremely difficult, although laryngeal mask airways (LMAs) have been used successfully (Naguib et al., 1986; Nargozian, 2004).
The Face
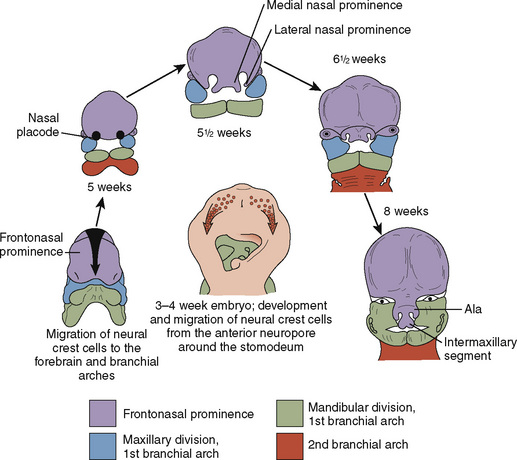
Just as the neurocranium forms the cranial vault and base, the viscerocranium forms the face and is derived mainly from cartilage of the first two branchial arches (Fig. 12-3). Ectodermally-derived neural crest cells of the developing 3- to 4-week-old embryo migrate to branchial arch mesoderm, and the face develops as a result of these massive cell migrations and their interactions. Those cells forming the frontonasal process are derived from the forebrain fold and migrate a relatively short distance as they pass into the nasal region. Those cells that form the mesenchyme of the maxillary and mandibular processes have a considerably longer distance to migrate, because they must move into the branchial arches. At 28 days postconception, the face barely shows its eventual relation to the five primordia from which it is derived: the frontonasal prominence, which is the cranial boundary of the primitive mouth (stomodeum); the paired maxillary prominences (the first branchial arch); and the paired mandibular prominences (also the first branchial arch).
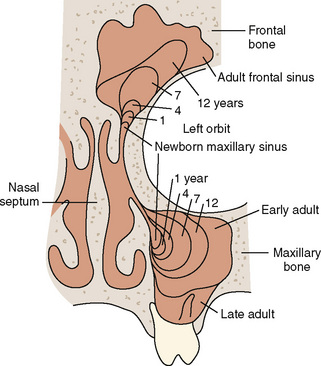
The paranasal sinuses begin developing at approximately 40 weeks of gestational age. The completion of turbinate development signals the beginning of sinus development, which continues until early adult life (Fig. 12-4). Although the exact function of the paranasal sinuses is not well understood, inflammatory, infectious, and neoplastic diseases of the sinuses are of major significance to the anesthesiologist, particularly if there is functional impairment before anesthesia and surgery. Sinus disorders are often comorbidities of asthma, immunoglobulin deficiencies, cystic fibrosis, or Kartagener’s syndrome.
The oral cavity—a structure without structures—where much of the anesthesiologist’s attention and skills are focused, has a complex developmental heritage. The mouth (stomodeum) appears as a slight depression in the surface ectoderm, separated from the oral cavity by the oropharyngeal membrane. This membrane ruptures at about 24 to 26 days’ gestation and the primitive foregut then communicates with the amniotic cavity. The involved germ layers are the endoderm internally and the ectoderm externally. The tongue surface arises primarily from first arch mesenchyme, with significant contributions from the third and fourth arches, hence its complex innervation by the facial nerve in the anterior two thirds and the hypoglossal nerve in the posterior one third (Fig. 12-5). The muscle bulk of the tongue arises primarily from occipital somites, explaining the hypoglossal nerve (XII) innervation and its susceptibility to injury from errant placement of dental rolls and pressure injury from overinflated LMA cuffs. The upper lip is formed by the merging of the maxillary prominences with the medial nasal prominences, with the lateral basal prominences forming the alae. The intermaxillary segment in the central portion of the upper lip area consists of a labial component (forming the philtrum), a maxillary component (associated with the four incisor teeth), and a palatal component (which becomes the primary palate).
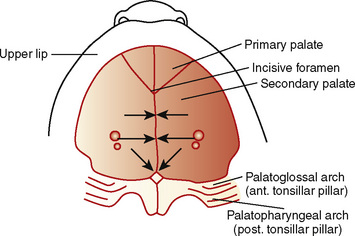
The palate divides the nasomaxillary complex from the oral cavity (Fig. 12-6). The palatal processes advance in a medial direction from the maxillary processes of the first branchial arch, fusing in the midline in an anterior-to-posterior sequence and uniting with the premaxilla and the developing nasal septum. The soft palate forms from continued growth of the posterior edges of these palatal processes, ending with the formation and fusion of the two halves of the uvula.
Clinical Correlation
Closure of the cleft palate may result in insufficient tissue for development of normal length or function of the soft palate and require a posterior pharyngeal flap. Velopharyngeal insufficiency is the cause of the hypernasal speech, nasal emission, and nasal turbulence (Sidman and Muntz, 2000).
The Branchial Apparatus
Branchial Arches
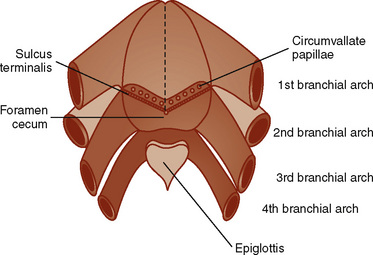
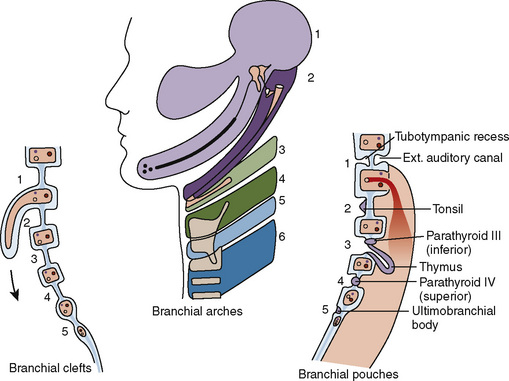
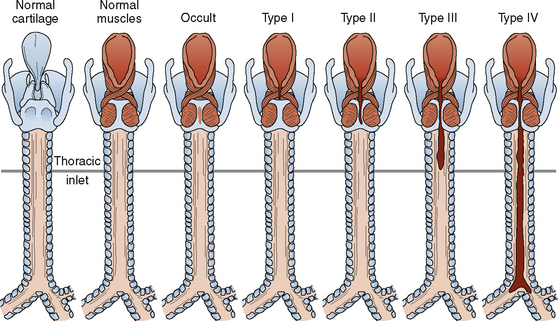
The branchial apparatus consists of four branchial arches visible on the surface of the embryo, as well as fifth and sixth arches that cannot be seen on the surface. Branchial pouches and clefts are likewise numbered craniocaudally (Fig. 12-7). The first branchial arch (Meckel’s) cartilage is the position of the future mandible, as well as the eventual malleus and incus. The second branchial arch cartilage produces the stapes, the styloid process, the stylohyoid ligament, and the superior portion of the body of the hyoid. The other branchial arch cartilages contribute to the inferior portion of the hyoid as well as the thyroid cartilage.
Branchial Pouches
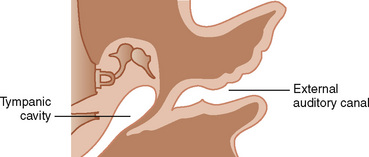
The first branchial pouch develops into the tubotympanic recess, becoming the auditory tube and the middle ear cavity (Fig. 12-8). The cavity of the second branchial pouch is largely obliterated as the palatine tonsil develops, but part of it remains as the tonsillar fossa. The endoderm of the second branchial pouch becomes the surface epithelium of the tonsil and the lining of its crypts, with the mesenchyme around the pouch differentiating into lymphoid tissue. The endoderm of the dorsal part of the third branchial pouches differentiates into the inferior parathyroids, and the ventral parts unite to become the thymus. The paired parathyroid glands develop from separate pouches, with one pair derived from the third and the other from the fourth branchial pouch. At the seventh week of development, the parathyroid glands migrate caudally from their respective branchial pouches, with the third pouch parathyroids moving more caudally than the parathyroids of the fourth pouch. Accessory parathyroid tissue may be left along the line of migration. The endoderm of the fourth branchial pouches differentiates into the superior parathyroid glands, and the ventral parts develop into the ultimobranchial bodies, the calcitonin-secreting portion of the thyroid.
Clinical Correlation
The thyroid begins as a thickening of the endoderm of the floor of the pharynx, in the midline between the first and second pouches, at the foramen cecum. A thin connection, the thyroglossal duct, remains attached to the oral cavity, and its point of attachment marks the origin of the thyroid gland. The thyroid descends along the thyroglossal duct and reaches the level of the first tracheal ring at about the seventh week of gestation (Fig. 12-9). The thyroglossal duct is then normally obliterated. Accessory thyroid tissue may be deposited anywhere along this path; on the other hand, failure of the thyroid to descend may result in a lingual thyroid.
The Larynx
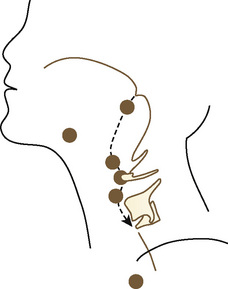
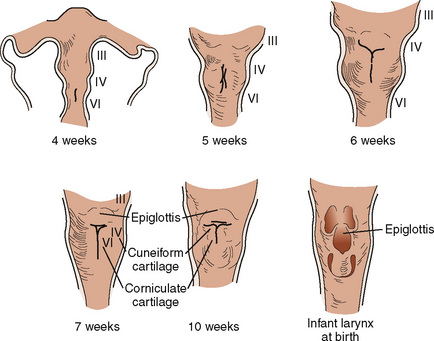
Development of the larynx begins at approximately 3 weeks of gestational age with the formation of the laryngotracheal tube from the ventral wall of the foregut. The laryngotracheal tube then grows caudally into the splanchnic mesoderm on the ventral surface of the foregut, dividing into the right and left lung buds. The epiglottis begins to form from the hypobranchial eminence of the third and fourth arches at approximately 30 to 32 days’ gestation. The aryepiglottic folds develop from the lateral boundaries of the fourth arch along a line from the hypobranchial eminence (epiglottis) to the arytenoid eminence of the sixth arch. Incomplete development at this stage may produce varying degrees of persistent laryngeal cleft (Fig. 12-10). A definite larynx may be seen by 41 days’ gestation. (Fig. 12-11).
Eckenhoff’s (1951) review of the anatomy of the pediatric larynx has influenced more than a generation of pediatric anesthesiologists with regard to the selection of endotracheal tube size based on the concept of the narrowest (fixed) portion of the upper airway being the level of the cricoid cartilage, or the “funnel-morphing-into-cylinder” concept. Litman et al. (2003) examined laryngeal shape in sedated children undergoing MRI and determined that under sedated conditions with tonic maintenance of laryngeal shape, it is more cylindric than funnel-shaped, as it is in adults, and that the narrowest portion of the airway is at the level of the vocal cords. Furthermore, they concluded that there is no change in this relationship from childhood to adulthood. This finding has been confirmed by video-bronchoscopic imaging in anesthetized and paralyzed children as well and provides an intriguing challenge to traditional teaching (see Chapter 3, Respiratory Physiology) (Dalal et al., 2008, 2009). The adjudication of these disparate concepts, as well as the confirmation in both reports that the cricoid opening is elliptic rather than circular in shape, with the narrowest transverse diameter, help support the transition over the last 10 to 15 years to the common use of cuffed endotracheal tubes with a smaller diameter rather than tightly (or “appropriately”) fitted, uncuffed endotracheal tubes. Moreover, advances in materials and design have allowed the development of thin-walled tubes with shorter, polyurethane cuffs, which provide an equivalent seal at a lower mucosal pressure (Dullenkopf et al., 2005). At this point, the increasingly routine use of cuffed endotracheal tubes, with appropriate care, appears to result in fewer repeated laryngoscopies and reintubations, less postintubation croup and less contamination in the operating room, and lower total fresh-gas flows.
Developmental physiology
The Upper Airway
Isono (2006) has reviewed the physiology of airway maintenance at the pharyngeal level and described the pharynx as a collapsible air-filled tube surrounded by soft tissues enclosed in a rigid box of bony structures, the mandible and vertebrae, with the lumen of the tube determined by the balance of the soft-tissue mass and the size of the surrounding rigid box. This simple approach explains the success of routine clinical airway maneuvers—continuous positive airway pressure (CPAP) serving as a pneumatic stent for the collapsed air-filled tube and surrounding soft tissue, and anterior displacement of the jaw serving to enlarge the rigid box. An oral airway displaces the base of the tongue anteriorly, thereby increasing the transpharyngeal luminal space and moving the base of the tongue away from the prevertebral bodies. Anesthetics, of course, depress the integrity of pharyngeal muscle tone through a variety of mechanisms, including the attenuation of neural input caused by the loss of consciousness and the decrease in tone of the diaphragm and intercostal musculature. The tongue and its muscular attachments such as the genioglossus, geniohyoid, sternohyoid, sternothyroid, and thyrohyoid are affected as well. All of this conspires to narrow the pharyngeal airway, more so in infants and small children than adults, especially during the first year of life (Isono et al., 2000). Adults are more equipped than children to defend against these effects, because they possess a more competent negative pressure reflex serving to augment pharyngeal tone, although the negative pressure reflex has been shown in infants younger than 1 year old as well (Thach et al., 1989; Horner et al., 1991). In addition, the progressive increase in pharyngeal cross-sectional area during the first year of life is positively influenced by the growth of the mandible and maxilla.
Pharyngeal airway obstruction has long been recognized as a significant component of the clinical entity called laryngospasm, and the application of CPAP, whether applied transnasally or transorally, depresses pharyngeal muscle tension and provides a mechanical pneumatic stent for the upper airway (Fink, 1956; Alex et al., 1987). This is a subtle component of the routine technique used by experienced pediatric anesthesiologists in applying gentle amounts of positive pressure during the induction phase, especially with infants and small children.
The fetus is an experienced swallower, well practiced from the age of 10 to 11 weeks’ gestation, with suckling following at 18 to 24 weeks’ gestation. This is fortunate, because coordination of this highly complex task is dependent on practice in utero and learning in pre- and postnatal life. However, breathing, even in utero, is a relatively late event, occurring at about 32 to 37 weeks’ gestational age, and premature infants show their lack of experience with significant discoordination between swallowing and breathing, as do children who are neurologically impaired (Goldson, 1987; Arvedson et al., 1994).

Full access? Get Clinical Tree