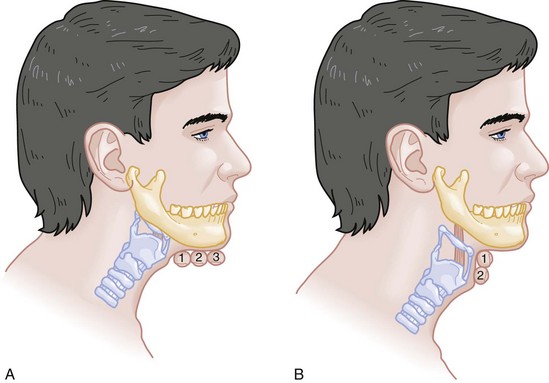
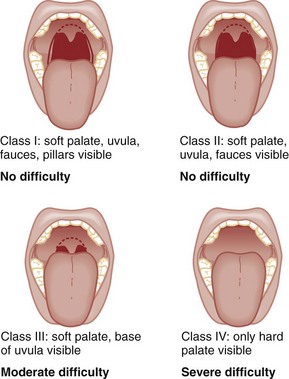
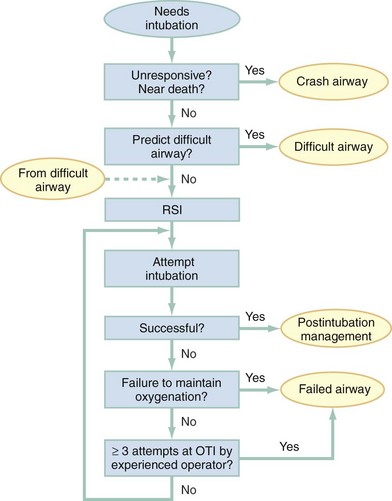
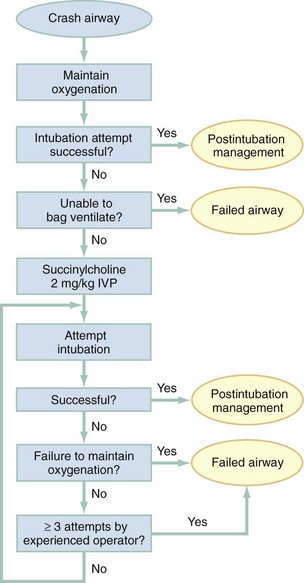
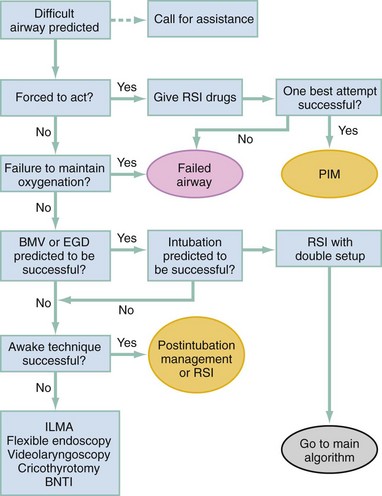
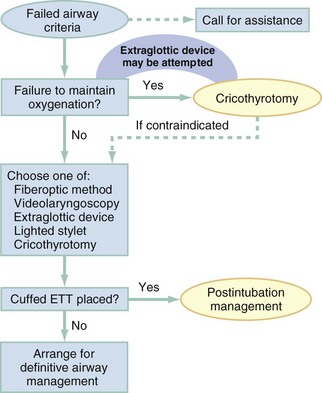
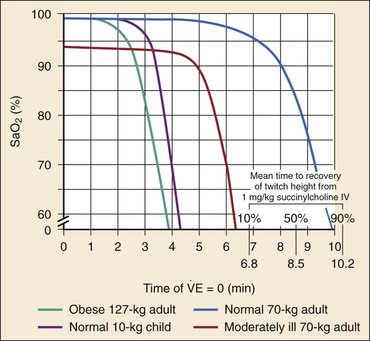
Chapter 1 A patent airway is essential for adequate ventilation and oxygenation. If the patient is unable to maintain an open airway, patency should be established by artificial means, such as repositioning, chin lift, jaw thrust, or insertion of an oral or nasal airway. Likewise, the patient must be able to protect against aspiration of gastric contents, which carries significant morbidity and mortality. Historically, the presence or absence of a gag reflex has been advocated as a reliable indicator of the patient’s ability to protect the airway, but the gag reflex is absent in 12 to 25% of normal adults, and there is no evidence that its presence or absence corresponds to airway protective reflexes or the need for intubation.1 The patient’s ability to swallow or handle secretions is a more reliable indicator of airway protection. The recommended approach is to evaluate the patient’s level of consciousness, the ability to phonate in response to voice command or query (which provides information about both the integrity of the upper airway and the level of consciousness), and the ability to manage his or her own secretions (e.g., pooling of secretions in the oropharynx, absence of swallowing spontaneously or on command.) In general, a patient who requires a maneuver to establish a patent airway or who easily tolerates an oral airway probably requires intubation for protection of that airway, unless a temporary or readily reversible condition, such as opioid overdose, is present. Regardless of the underlying cause, the need for mechanical ventilation generally mandates intubation. External mask devices increasingly have been used to provide assisted mechanical ventilation without intubation (see Chapter 2), but despite these advances, most patients who need assisted ventilation or positive pressure to improve oxygenation require intubation.2 Certain conditions indicate the need for intubation even in the absence of frank airway, ventilatory, or oxygenation failure. These conditions are characterized by a moderate to high likelihood of inevitable intubation to facilitate the patient’s workup and treatment or predictable airway deterioration that would require intervention. Intubation may be indicated relatively early in the course of certain overdoses. Although the patient initially may be protecting the airway and exchanging gas adequately, intubation often is advisable to guard against the strong likelihood of clinical deterioration, which can occur after the initial phase of care when the patient no longer is closely observed. Significant multiple trauma, with or without head injury, may be an indication for intubation even if the patient is ventilating normally through a patent airway and has adequate oxygen levels.3,4 Multiple trauma with hypotension, an open femur fracture, and diffuse abdominal tenderness warrant early intubation even if the patient is initially awake and alert without airway injury or hypoxemia. Aggressive resuscitation, pain control, the need for invasive procedures and imaging outside of the emergency department (ED), and inevitable operative management argues strongly for early airway control. In addition, a patient with penetrating neck trauma may have a patent airway and adequate gas exchange. Nevertheless, early intubation is advisable with any evidence of vascular or direct airway injury because these patients tend to deteriorate and increasing hemorrhage or swelling in the neck tend to both compromise the airway and confound later attempts at intubation.5,6 Identification of the Difficult Airway In most patients, intubation is technically easy and straightforward. In large ED studies, overall intubation failure rates resulting in rescue cricothyrotomy are 0.9% for all intubations and 1.7% for trauma patients.7–9 Intubation failure occurs in approximately 1 in 200 to 1 in 2000 elective general anesthesia cases.10 Bag-mask ventilation (BMV) is difficult in approximately 1 in 50 general anesthesia patients and impossible in approximately 1 in 600.11,12 BMV is difficult, however, in up to one third of patients in whom intubation failure occurs, and difficult BMV makes the likelihood of difficult intubation four times higher and the likelihood of impossible intubation 12 times higher.11,12 The combination of failure of intubation and failure of BMV in elective anesthesia practice is estimated to be exceedingly rare: 1 in 5000 to 1 in 20,000 elective anesthesia patients.12,13 These numbers cannot be extrapolated to populations of ED patients who are acutely ill or injured and for whom intubation is both urgent and unavoidable. Although patient selection cannot occur (as with a preanesthetic visit), a preintubation analysis of factors predicting difficult intubation can provide the operator with the information necessary to formulate a safe and effective plan for intubation. Preintubation assessment should evaluate the patient for difficult intubation, difficult BMV, difficult ventilation with an extraglottic device (EGD; see later discussion), and difficult cricothyrotomy. Knowledge of all four domains is crucial to successful planning.10 Most of the difficult airway markers discussed in the anesthesia and emergency medicine literature have not been scientifically validated.14 Nevertheless, a methodical approach can be used to evaluate the patient, based on the accepted hallmarks of difficult intubation by direct laryngoscopy. Videolaryngoscopy, on the other hand, so rarely fails to provide excellent laryngeal visualization that characterization of difficult videolaryngoscopy predictors may not be possible. When direct laryngoscopy is planned, a standard screening process for difficulty should be undertaken with every patient. It is probably useful to perform this screen when videolaryngoscopy is planned, as well, because it may help to identify challenges, such as a very large tongue or poor mouth access. One such approach uses the mnemonic LEMON (Box 1-1).10,15 L—Look externally. The patient first should be examined for external markers of difficult intubation, which are determined based simply on the intubator’s clinical impression. For example, the severely bruised and bloodied face of a combative trauma patient, immobilized in a cervical collar on a spine board, should (correctly) invoke an immediate appreciation of anticipated difficult intubation. Subjective clinical judgment can be highly specific (90%) but insensitive and so should be augmented by other evaluations.12 E—Evaluate 3-3-2. The second step in the evaluation of the difficult airway is to assess the patient’s airway geometry to determine his or her suitability for direct laryngoscopy. Glottic visualization with a direct laryngoscope requires that the mouth open adequately, that the submandibular space be adequate to accommodate the tongue, and that the larynx be positioned low enough in the neck to be accessible. These relationships have been explored in various studies by external measurement of mouth opening, oropharyngeal size, neck movement, and thyromental distance.16 The “3-3-2 rule” is an effective summary of these assessments.10,15 The 3-3-2 rule requires that the patient be able to place 3 of his or her own fingers between the open incisors, 3 of his or her own fingers along the floor of the mandible beginning at the mentum, and 2 fingers from the laryngeal prominence to the floor of the mandible (Fig. 1-1). A patient with a receding mandible and high-riding larynx can be impossible to intubate using direct laryngoscopy because the operator cannot adequately displace the tongue and the angle that must be overcome for a direct view of the glottic aperture is too acute. In practice, the operator compares the size of his or her fingers with the size of the patient’s fingers and then performs the three tests. M—Mallampati scale. Oral access is assessed with the Mallampati scale (Fig. 1-2). Visibility of the oral pharynx ranges from complete visualization, including the tonsillar pillars (class I), to no visualization at all, with the tongue pressed against the hard palate (class IV). Class I and class II predict adequate oral access, class III predicts moderate difficulty, and class IV predicts a high degree of difficulty.16,17 A meta-analysis confirmed that the four-class Mallampati score performs well as a predictor of difficult laryngoscopy (and, less so, of difficult intubation) but that the Mallampati score alone is not a sufficient assessment tool.18 A Mallampati score may have to be improvised with the direct laryngoscope blade as a tongue depressor in obtunded or uncooperative patients.19 O—Obstruction or obesity. Upper airway (supraglottic) obstruction may make visualization of the glottis, or intubation itself, mechanically impossible. Conditions such as epiglottitis, head and neck cancer, Ludwig’s angina, neck hematoma, or glottic polyps can compromise laryngoscopy, passage of the endotracheal tube (ETT), BMV, or all three. Examine the patient for airway obstruction and assess the patient’s voice to satisfy this evaluation step. There is conflicting evidence regarding whether obesity is itself an independent marker of difficult intubation or whether patients with obesity simply are more likely to have other markers of difficult intubation.20,21 Regardless, obese patients generally are more difficult to intubate than their nonobese counterparts, and preparations should account both for this and for the more rapid oxyhemoglobin desaturation and increased difficulty with ventilation using bag and mask or an EGD (see later) that will occur. N—Neck mobility. Neck mobility is desirable for any intubation technique and is essential for positioning the patient for optimal direct laryngoscopy. Neck mobility is assessed with the patient’s flexion and extension of the head and neck through a full range of motion. Neck extension is the most important motion, and simple extension may be as effective as the “sniffing” position in achieving an optimal laryngeal view.22 One study found that the “extension-extension” position, in which the neck is extended on the body (opposite of the flexion used for the sniffing position) with the head extended on the neck, provides superior laryngeal views to the sniffing position.23 Modest limitations of motion do not seriously impair direct laryngoscopy, but severe loss of motion, as can occur in ankylosing spondylitis or rheumatoid arthritis, for example, may render direct laryngoscopy impossible. Cervical spine immobilization in trauma artificially reduces cervical spine mobility, but direct laryngoscopy is still highly successful in this group of patients.7 Identification of a difficult intubation does not preclude use of an RSI technique. The crucial determination is whether the clinician judges that the patient has a reasonable likelihood of intubation success, despite the difficulties identified, and that ventilation with a bag and mask or an EGD will be successful in the event that intubation fails (hence the value of the BMV and EGD assessments; see Boxes 1-2 and 1-3). Attributes of difficult BMV have largely been validated and can be summarized with the mnemonic MOANS (see Box 1-2).10–12 Mask seal compromise or difficulty; Obstruction (particularly supraglottic obstruction, but it can be present anywhere in the airway) or Obesity (because of redundant upper airway tissues, chest wall weight, and resistance of abdominal mass); advanced Age (best judged by the physiologic appearance of the patient, but age older than 55 years increases risk); edentulousness (“No teeth”), which independently interferes with mask seal; and “Stiffness” or resistance to ventilation (e.g., asthma, chronic obstructive pulmonary disease, pulmonary edema, restrictive lung disease, term pregnancy) may each contribute to increased difficulty with BMV. The difficulty with BMV of the edentulous patient is the basis of the advice “Teeth out to intubate, teeth in to ventilate.” Multiple studies have validated this advice.24 A new approach involves placing the mask inside the patient’s lower lip. This may limit air leak in patients without teeth and eliminates the risk of aspiration associated with dental prosthetics or rolled gauze (Fig. 1-3).25 Difficult BMV is not uncommon, but with proper technique it usually is successful. A recent review of more than 50,000 patients undergoing elective anesthesia found that impossible BMV was exceptionally rare (0.2%) and was associated with neck changes secondary to radiation therapy, presence of a beard, male sex, history of sleep apnea, and a Mallampati class III or IV airway. Impossible BMV was five times more likely if one of these attributes was present and 25 times more likely with four or more.26 Placement of an EGD, such as a laryngeal mask airway (LMA), a Combitube, or a similar upper airway device, often can convert a “can’t intubate, can’t oxygenate” situation to a “can’t intubate, can oxygenate” situation, which allows time for rescue of a failed airway (see following section.) Difficulty achieving placement or ventilation with an EGD is predicted by the mnemonic RODS. Fortunately, if the clinician has already performed the LEMON and MOANS assessments, only the D for distorted anatomy remains to be evaluated (see Box 1-3). EGDs are placed blindly and have either a mask or a balloon structure that, when inflated, obstructs the oropharynx proximally and the esophageal inlet distally, permitting indirect ventilation. Distorted upper airway anatomy can result in a poor seal and ineffective ventilation. Difficult cricothyrotomy can be anticipated whenever there is disturbance of the ability to locate and access the landmarks of the anterior airway via the neck and is remembered by the SMART mnemonic (Box 1-4). Prior surgery; hematoma, tumor, or abscess; scarring (as from radiation therapy or prior injury); local trauma; obesity; edema; or subcutaneous air each has the potential to make cricothyrotomy more difficult. Perform an examination for the landmarks needed to perform cricothyrotomy as part of the preintubation difficult airway assessment of the patient. The actual degree to which an intubation is “difficult” is highly subjective, and quantification is challenging. The most widely used system for grading laryngoscopic view of the glottis is that of Cormack and Lehane (CL),26a which grades laryngoscopy according to the extent to which laryngeal and glottic structures can be seen. In grade 1 laryngoscopy, all or nearly all of the glottic aperture is seen. Grade 2 laryngoscopy visualizes only a portion of the glottis (arytenoid cartilages alone or arytenoid cartilages plus part of the vocal cords). Grade 3 laryngoscopy visualizes only the epiglottis. In grade 4 laryngoscopy, not even the epiglottis is visible. Research conducted on elective anesthesia patients undergoing direct laryngoscopy suggests that true grade 4 laryngoscopy, which is associated with impossible intubation, occurs in less than 1% of patients. Grade 3 laryngoscopy, which represents extreme intubation difficulty, is found in less than 5% of patients. Grade 2 laryngoscopy, which occurs in 10 to 30% of patients, can be subdivided further into grade 2a, in which arytenoids and a portion of the vocal cords are seen, and grade 2b, in which only the arytenoids are seen. Intubation failure occurs in 67% of grade 2b cases but in only 4% of grade 2a cases.27 Outside of the operating room, the rate of difficulty may be higher. In a recent review of emergency adult inpatient intubations, as many as 10% were considered difficult (either a grade 3 or 4 CL direct view or more than three attempts required).28 The incidence of difficult ED intubations is unknown but is likely much higher, with some estimates between 4 and 26%.9,29 Approximately 80% of all grade 2 laryngoscopies are grade 2a; the rest are grade 2b. A grade 1 view is associated with virtually 100% intubation success. An alternative system, POGO (percentage of glottic opening), also has been proposed and validated but is not widely used or studied.30 The most serious complication of endotracheal intubation is unrecognized esophageal intubation with resultant hypoxic brain injury. Although direct visualization of the ETT passing through the vocal cords generally is a reliable indicator of tracheal intubation, such clinical anatomic observations are fallible, and additional means are required to ensure correct placement of the tube within the trachea. Traditional methods, such as chest auscultation, gastric auscultation, bag resistance, exhaled volume, visualization of condensation within the ETT, and chest radiography, all are prone to failure as means of confirming tracheal intubation.31 Other clinical techniques are readily available for detecting tracheal or esophageal intubation. Immediately after intubation, the intubator should apply an end-tidal carbon dioxide (ETCO2) detection device to the ETT and assess it through six manual ventilations. Disposable, colorimetric ETCO2 detectors are highly reliable, convenient, and easy to interpret, indicating adequate CO2 detection by color change (Figs. 1-4 and 1-5). ETCO2 detection is highly reliable in determining tracheal and esophageal intubation in patients with spontaneous circulation.32 These devices indicate the carbon dioxide (CO2) content in exhaled air either qualitatively or quantitatively. The persistence of detected CO2 after six manual breaths indicates that the tube is within the airway, although not necessarily within the trachea. CO2 is detected with the tube in the mainstem bronchus, the trachea, or the supraglottic space. Correlation of ETCO2 detection with the depth markings on the ETT (particularly important in pediatric patients) confirms tracheal placement. Rarely, BMV before intubation or ingestion of carbonated beverages may lead to release of CO2 from the stomach after esophageal intubation, causing a transient false indication of tracheal intubation. Washout of this phenomenon occurs within six breaths, however, so persistence of CO2 detection after six breaths indicates tracheal intubation. Although colorimetric ETCO2 measurement is highly sensitive and specific for detecting esophageal intubation, caution is required for patients with cardiopulmonary arrest. Insufficient gas exchange may hamper CO2 detection in the exhaled air, even when the tube is correctly placed within the trachea.32 In patients with cardiopulmonary arrest, a CO2 level greater than 2%, which is the threshold for color change on colorimetric capnometers, should be considered definitive evidence of correct ETT placement, but the absence of such CO2 cannot be used reliably as an indicator of esophageal intubation. This circumstance arises in approximately 25 to 40% of intubated cardiac arrest patients.32,33 In all other patients, absence of CO2 detection indicates failure to intubate the trachea, and rapid reintubation is indicated. When possible, continuous quantitative capnography is more accurate and yields more information than capnometry (including colorimetric devices). When ETCO2 detection is not possible, tracheal tube position can be confirmed with other techniques. One novel approach involves bedside ultrasound. In both live patient and cadaver studies, ultrasonography performed over the cricothyroid membrane or upper trachea accurately confirmed ETT position in the trachea, especially during the act of intubation.34,35 Bulb or syringe aspiration devices may be used in patients with cardiac arrest who have no detectable CO2, but although such devices are highly reliable at detecting esophageal intubation (sensitivity > 95%), false positives, in which a correctly placed tracheal tube is incorrectly identified as esophageal, can occur in up to 25% of cardiac arrest patients.32 Aspiration devices may be useful in the out-of-hospital setting when poor lighting hampers colorimetric ETCO2 determination. They also are good backup devices when cardiac arrest confounds attempts to assess placement with ETCO2. Detection of expired CO2 is more reliable and should be considered the standard for confirmation of tracheal placement of an ETT and for early detection of accidental esophageal intubation. Aspiration devices have a valuable but secondary role. Accordingly, ETCO2 detection, with either aspiration or ultrasound techniques as backup, should be considered the primary means of ETT placement confirmation. Secondary means include physical examination findings, oximetry, and radiography. The examiner should auscultate both lung fields and the epigastric area. Pulse oximetry is indicated as a monitoring technique in all critically ill patients, not just those who require intubation. Oximetry is useful in detecting esophageal intubation but may not show a decreasing oxygen saturation for several minutes after a failed intubation because of the oxygen reservoir (preoxygenation) created in the patient before intubation.36 Although chest radiography is universally recommended after ETT placement, its primary purpose is to ensure that the tube is well positioned below the cords and above the carina. A single anteroposterior chest radiograph is not sufficient to detect esophageal intubation, although esophageal intubation may be detected if the ETT is clearly outside the air shadow of the trachea. In cases in which doubt persists, a fiberoptic scope can be passed through the ETT to identify tracheal rings, another “gold standard” for confirmation of tracheal placement. Algorithms for emergency airway management have been developed and provide a useful guide, both for planning intubation and for rescue in the event of intubation failure. The algorithm assumes that a decision to intubate has been made and outlines such an approach. The approach is predicated on two key determinations that must be made before active airway management is begun (Fig. 1-6). The first determination is whether the patient is in cardiopulmonary arrest or a state of near arrest and is predicted to be unresponsive to direct laryngoscopy. Such a patient (agonal, near death, in circulatory collapse) is called a “crash airway” patient for the purposes of emergency airway management and is treated with the crash airway algorithm by an immediate intubation attempt without use of drugs, supplemented by a single, large dose of succinylcholine if the attempt to intubate fails and the patient is thought not to be sufficiently relaxed (Fig. 1-7). Next, it must be determined whether the patient represents a difficult intubation as determined by the LEMON, MOANS, RODS, and SMART evaluations, and if so, the difficult airway algorithm is used (Fig. 1-8). For patients who require emergency intubation but who have neither a crash airway nor a difficult airway, we recommend RSI. RSI provides the safest and quickest method of achieving intubation in such patients.7,14,37,38 After administration of the RSI drugs, intubation attempts are repeated until the patient is intubated or a failed intubation is identified. If more than one intubation attempt is required, oxygen saturation is monitored continuously, and if saturation falls to 90% or less, BMV is performed until saturation is recovered for another attempt. If the clinician cannot maintain oxygen saturation despite optimal use of BMV or an EGD, a failed airway exists. This is referred to as a “can’t intubate, can’t oxygenate” situation. In addition, if three attempts at laryngoscopy have been unsuccessful, a failed airway exists because subsequent attempts at laryngoscopy by the same clinician are unlikely to succeed. The three failed laryngoscopy attempts are defined as attempts by an experienced clinician using the best possible patient positioning and technique. If available, videolaryngoscopes should be used for one or all of these attempts. Also, if the clinician ascertains after even a single attempt that intubation will be impossible (e.g., grade 4 laryngoscopic view with a direct laryngoscope despite optimal patient positioning and use of external laryngeal manipulation), and no alternative laryngoscope (e.g., videolaryngoscope) is available, a failed airway is present. The failed airway is managed according to the failed airway algorithm (Fig. 1-9). The perception of a difficult airway is relative, and many emergency intubations could be considered “difficult.” The judgment regarding whether to treat the airway as a typical emergency airway or whether to use the difficult airway algorithm is based on the degree of perceived difficulty, operator experience, the airway management devices available, and the individual circumstances of the case.39 The LEMON, MOANS, RODS, and SMART assessments provide a systematic framework to assist in identifying the potentially difficult airway. When preintubation evaluation identifies a potentially difficult airway (see Fig. 1-8), the approach is based on the premise that NMBAs generally should not be used unless the clinician believes that (1) intubation is likely to be successful and (2) oxygenation via BMV or EGD is likely to be successful if a first intubation attempt does not succeed and oxygenation is required.10 The one exception to this recommendation occurs in the “forced to act” scenario. Therefore, in the difficult airway algorithm, the first determination is whether the operator is “forced to act.” If so, RSI drugs are given, a best attempt at laryngoscopy is undertaken, and, if intubation is not successful, the airway is considered failed and the operator moves immediately to the failed airway algorithm. In the vast majority of difficult airway situations, however, the operator is not forced to act, and the first step is to ensure that oxygenation is sufficient to permit a planned, orderly approach (see Fig. 1-8). If oxygenation is inadequate and cannot be made adequate by supplementation with bag and mask, the airway should be considered a failed airway. Whereas inadequate oxygenation should be defined on a case-by-case basis, oxygenation saturation at or below 90% is the accepted threshold because this represents the point at which hemoglobin undergoes a conformational change, more readily releasing oxygen and increasing the pace of desaturation. Oxyhemoglobin saturations in the 80s, if holding steady, might be considered adequate in some circumstances, particularly if the patient is chronically hypoxemic. When oxygenation is inadequate, the failed airway algorithm should be used because the predicted high degree of intubation difficulty, combined with failure to maintain oxygen saturation, is analogous to the “can’t intubate, can’t oxygenate” situation. When oxygenation is adequate, however, the next consideration is whether RSI is appropriate, on the basis of the operator’s assessment of the likelihood of (1) successful ventilation with a bag and mask or an EGD in the event intubation is unsuccessful, and (2) the likelihood of successful intubation by laryngoscopy. If the operator judges laryngoscopy likely to succeed and is confident of the ability to oxygenate even if intubation fails, then RSI is performed. In such cases a double setup can be used in which RSI is performed and preparations simultaneously are undertaken for rescue cricothyrotomy or another rescue technique. If RSI is not advisable and time allows, an awake technique can be used. In this context, awake means that the patient continues to breathe and is able to cooperate with caregivers. Usually the technique involves sedation and topical anesthesia, ideally preceded by a drying agent such as glycopyrrolate. The awake technique often is direct or flexible or rigid videolaryngoscopy, assisted by topical anesthesia and sedation (comparable to that for a painful procedure). If the glottis is adequately visualized, the patient can be intubated at that time, or, in a stable difficult airway situation, the clinician may proceed with planned RSI, now assured of intubation success. If the awake laryngoscopy is unsuccessful, the patient is intubated with any of numerous techniques shown in the last box in Figure 1-8. For each of these methods, the patient is kept breathing but variably sedated and anesthetized, and each of the methods results in placement of a cuffed ETT in the trachea. The choice among these methods depends on clinician experience and preference, device availability, and patient attributes. Management of the failed airway is dictated by whether the patient can be oxygenated.10 If adequate oxygenation cannot be maintained, the rescue technique of first resort is cricothyrotomy (see Fig. 1-9). Multiple attempts at other methods in the context of failed oxygenation delay cricothyrotomy and place the patient at increased risk for hypoxic brain injury. If an alternative device (i.e., an EGD such as an LMA or Combitube) is immediately available, however, and the operator judges it to be an appropriate device for the patient’s anatomy, an attempt can be made to use it simultaneously with preparations for immediate cricothyrotomy, as long as initiation of cricothyrotomy is not delayed. Only a single attempt with the EGD is recommended in this circumstance. Although many techniques are available for intubation of the emergency patient, four methods are most common, with RSI being the most frequently used in nonarrested patients.7–9,40 RSI is the cornerstone of modern emergency airway management and is defined as the virtually simultaneous administration of a potent sedative (induction) agent and an NMBA, traditionally succinylcholine, for the purpose of endotracheal intubation. This approach provides optimal intubating conditions and has long been believed to minimize the risk of aspiration of gastric contents. A systematic review of the literature in 2007 failed to prove that RSI results in a lower incidence of aspiration than other techniques, but the authors correctly noted that virtually no studies have ever been designed to measure this precise endpoint.41 RSI is nevertheless the most widely used technique for emergency intubation of patients without identifiable difficult airway attributes.7–9 The central concept of RSI is to take the patient from the starting point (e.g., conscious, breathing spontaneously) to a state of unconsciousness with complete neuromuscular paralysis, then to achieve intubation without interposed assisted ventilation. The risk of aspiration of gastric contents is felt to be significantly higher for patients who have not fasted before induction. Application of positive-pressure ventilation can cause air to pass into the stomach, resulting in gastric distention and likely increasing the risk of regurgitation and aspiration.42 The purpose of RSI is to avoid positive-pressure ventilation until the ETT is placed correctly in the trachea with the cuff inflated. This requires a preoxygenation phase, during which the nitrogen reservoir in the functional residual capacity in the lungs is replaced with oxygen, permitting at least several minutes of apnea (see later discussion) in the normal adult before oxygen desaturation to 90% ensues (Fig. 1-10).36 Use of RSI also facilitates successful endotracheal intubation by causing complete relaxation of the patient’s musculature, allowing better access to the airway.37,38,43 Finally, RSI permits pharmacologic control of the physiologic responses to laryngoscopy and intubation, mitigating potential adverse effects. These effects include further intracranial pressure (ICP) increase in response to the procedure and to the sympathetic discharge resulting from laryngoscopy (Box 1-5).44 RSI is a series of discrete steps, and every step should be planned (Box 1-6).45 Preparation.: In the initial phase the patient is assessed for intubation difficulty (unless this has already been done), and the intubation is planned, including determining dosages and sequence of drugs, tube size, and laryngoscope type, blade, and size. Drugs are drawn up and labeled. All necessary equipment is assembled. All such patients require continuous cardiac monitoring and pulse oximetry. At least one and preferably two good-quality intravenous lines should be established. Redundancy is always desirable in case of equipment or intravenous access failure. Preoxygenation.: Administration of 100% oxygen for 3 minutes of normal, tidal volume breathing in a normal, healthy adult establishes an adequate oxygen reservoir to permit 8 minutes of apnea before oxygen desaturation to less than 90% occurs (see Fig. 1-10).36 Additional preoxygenation does not improve arterial oxygen tension. The time to desaturation to less than 90% in children, obese adults, late-term pregnant women, and patients with significant comorbidity is considerably less.46 Desaturation time also is reduced if the patient does not inspire 100% oxygen.47 Nevertheless, adequate preoxygenation usually can be obtained, even in ED patients, to permit several minutes of apnea before oxygen desaturation to less than 90% occurs. In children and adults, preoxygenation is essential to the “no bagging” approach of RSI. If time is insufficient for a full 3-minute preoxygenation phase, eight vital capacity breaths with high-flow oxygen can achieve oxygen saturations and apnea times that match or exceed those obtained with traditional preoxygenation.48 Desaturation time in obese patients can be prolonged by preoxygenating in the head up position and by continuing supplemental oxygen (by nasal cannula) after motor paralysis.49,50 Oxygen saturation monitors permit earlier detection of desaturation during laryngoscopy, but preoxygenation remains an essential step in RSI. Pretreatment.: During this phase, drugs are administered 3 minutes before administration of the succinylcholine and induction agent to mitigate the effects of laryngoscopy and intubation on the patient’s presenting or comorbid conditions. Intubation is intensely stimulating and results in sympathetic discharge (the reflex sympathetic response to laryngoscopy [RSRL]), elevation of ICP in patients with ICP disturbance, and reactive bronchospasm. Bradycardia can occur in children, particularly children younger than 1 year, but appears multifactorial, likely involving underlying parasympathetic tone, parasympathetic discharge in response to airway instrumentation, and perhaps some contributory effect of succinylcholine. Pretreatment focuses on three main objectives in certain at-risk patients. The three groups of patients at risk are those with reactive airway disease, elevated ICP, or a cardiovascular or neurovascular condition for which an acute elevation in blood pressure and heart rate might be hazardous. Patients with reactive airway disease often experience a worsening of their bronchospasm when intubated. Controversy exists regarding whether albuterol alone, lidocaine alone, or both drugs together are effective in reducing this intubation-related bronchospasm.51,52 Asthmatic patients being intubated in the ED for status asthmaticus will have received albuterol before intubation, and there is conflicting evidence regarding whether lidocaine has any additive protective effect in these situations. Pending larger studies, it is reasonable to administer lidocaine (1.5 mg/kg) as a pretreatment drug. When an asthmatic patient is being intubated for a condition other than acute asthma (e.g., trauma), nebulized albuterol, intravenous lidocaine, or both should be given before intubation, if possible. Patients with significant cardiovascular disease (e.g., ischemic coronary disease) who are being intubated in the ED may benefit from the administration of the synthetic opioid fentanyl in a dose of 3 µg/kg to blunt the release of catecholamines in response to airway manipulation. Similarly, patients with intracranial hemorrhage, elevated ICP, or marked hypertension may benefit from fentanyl (3 µg/kg) to mitigate the sympathetic induced rises in blood pressure. Finally, lidocaine may have a role in intracranial hypertension; however, the extent to which it can attenuate rises in ICP is controversial. Although there is some evidence that patients with elevated ICP may experience less exacerbation of the ICP during intubation if they are pretreated with lidocaine (1.5 mg/kg), results are not conclusive.53,54 Nevertheless, there is evidence supporting the physiologic effects of these agents even though outcome data are lacking. Individualization is necessary, and critical time should not be lost administering pretreatment drugs if the patient requires immediate intubation. Despite the lack of outcome studies, considerable inferential evidence supports this approach, and these agents, particularly fentanyl, probably provide protection for vulnerable patients against the adverse hemodynamic and intracranial effects of laryngoscopy and intubation. Although many variations are possible for pretreatment regimens in various conditions, pretreatment can be simplified to these three basic indications (see Box 1-5).
Airway
Pathophysiology
Failure to Maintain or Protect the Airway
Failure of Ventilation or Oxygenation
Anticipated Clinical Course
Clinical Features
Difficult Direct Laryngoscopy: LEMON
Difficult Bag-mask Ventilation: MOANS
Difficult Extraglottic Device Placement: RODS
Difficult Cricothyrotomy: SMART
Measurement and Incidence of Intubation Difficulty
Confirmation of Endotracheal Tube Placement
Management
Difficult Airway
Failed Airway
Therapeutic Modalities
Rapid Sequence Intubation

Full access? Get Clinical Tree


Airway
Only gold members can continue reading. Log In or Register to continue