Fig. 23.1
CTTRY. Procedural images of CTTRY picture are shown. A thoracic surgeon carefully manually traces the bronchi, pulmonary vessels, and tumor on CT images. The pulmonary arteries are shown in red, the pulmonary veins in blue, the bronchi in yellowish green, and the tumors in green
The cylindrical numeric data on the three-dimensional images of the bronchi, pulmonary vessels, tumor, and pulmonary lobes are reconstructed as 3D graphic images with the use of a freeware version of Metasequoia LE (http://metaseq.net/; Tetraface Inc, Tokyo, Japan), a free software package (Fig. 23.2). This software allows 3D images of the lung to be modified, and 3D images of the pulmonary vessels and bronchi to be transected and moved, thereby simulating surgery.
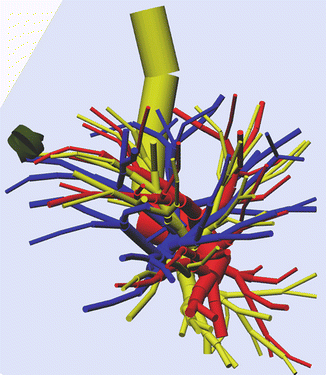

Fig. 23.2
Reconstructed 3D images of the lung using a freeware version of Metasequoia LE (http://metaseq.net/), a free software package
The pulmonary blood vessels and the bronchi are depicted as cylinders, which is adequate for understanding the anatomic spatial relations at the time of surgery. Because the data are simple, images of the pulmonary blood vessels, bronchi, and lung can be modified, transected, and moved, even on a notebook personal computer. Because CT scanning is performed during inhalation, the resulting 3D images are also in the inhalation phase. However, the 3D images can be modified to prepare 3D images of the collapsed lung during differential lung ventilation. On the other hand, when 3D images are reconstructed from conventional contrast-enhanced CT scans, it is difficult to separately depict arteries and veins. Two-stage images obtained in different phases have been reported to be useful, but exposure to radiation increases. Separate imaging of arteries and veins in different phases is generally performed to distinguish arteries from veins in the lung. However, this also requires an increased dose of contrast agents. In this respect, CTTRY allows 3D images to be reconstructed without using contrast media and produces very accurate images because reconstructed images are manually confirmed by comparing the images with CT scans [5–8].
Thoracoscopic Lung Segmentectomy
Surgery, radiotherapy, and chemotherapy are the mainstays of treatment for lung cancer. The therapeutic effectiveness of radiotherapy and chemotherapy remains unsatisfactory, and surgery is the standard treatment for clinical early-stage lung cancer.
Improved performance of diagnostic imaging devices and routine use of CT have dramatically increased the detection rate of small lung cancers. However, even small tumors can be associated with advanced disease. Therefore, parenchymal resection of at least one lobe with extensive hilar and mediastinal lymph-node dissection has become the standard surgical treatment throughout the world.
Since the later half of the 1970s, segmentectomy and partial resection have been indicated as limited surgery for compromised hosts with lung cancer. However, a randomized clinical trial comparing lobectomy with limited surgery in patients with clinical stage T1N0M0 lung cancer, reported by Ginsberg et al. in 1995, found that limited surgery, segmentectomy, or wedge resection were associated with higher rates of local recurrence and poorer outcomes as compared with lobectomy [9]. They concluded that lobectomy should be performed in patients who can tolerate the procedure, even for small lung cancers.
In Japan, which has established a national health checkup system, the proportion of patients who present with small and early-stage lung cancer associated with ground-glass opacity (GGO) on CT is higher than that in the USA. Consequently, many Japanese investigators reported that there is no distinct difference in outcomes between lobectomy and limited surgery. The surgical procedure of choice for early-stage, small lung cancers is thus shifting from lobectomy to segmentectomy, and techniques for limited surgery have received considerable attention.
As for the surgical approach, studies comparing open surgery with thoracoscopic surgery have yet to adequately demonstrate the superiority of thoracoscopic surgery in terms of invasiveness, safety, or curability. However, there is apparently no difference between these procedures in complications, local recurrence rate, length of hospital stay, or 5-year survival rate. Many studies thus reported that thoracoscopic surgery is a viable option [2, 3, 6].
Because lung resection can directly cause functional disorders, minimally invasive surgery should be performed while maintaining good outcomes. Thoracoscopic surgery can decrease postoperative pain and hasten recovery. Thoracoscopic surgery requires a small incision and is associated with less postoperative pain if no rib or muscle is transected. It is therefore considered minimally invasive surgery.
Recently, thoracoscopic segmentectomy with a small thoracic incision and minimal resection of lung volume has been increasingly performed as limited surgery in patients with small peripheral lung cancers, metastatic lung tumors located in the central portion of the lung, and impaired pulmonary function. Partial resection of lung has the smallest range of resection and can be performed thoracoscopically. However, partial resection of lung has limited indications and is not suited for resection of lesions located near the hilum. Another drawback of partial resection is the inability to evaluate lymph-node metastasis. Lung segmentectomy is associated with a smaller resection volume than lobectomy and allows resected tissue to be easily removed through a small incision, retaining the advantages of thoracoscopic surgery. Segmentectomy was initially used to manage benign lung tumors, infections, and inflammatory diseases, but was then performed as limited surgery in some poor-risk patients with lung cancer [2, 3]. The increased detection of early-stage small lung cancers and of peripheral lung cancer with a high air content then led to an increased number of patients who could be curatively treated by limited surgery, and the role of limited surgery was reconsidered. Survival rates after segmentectomy for small peripheral lung cancers 2 cm or less in diameter that were resectable by lobectomy were reported to be similar to those after lobectomy [2]. If preoperative imaging studies are strictly performed and the presence of surgical N0 disease is confirmed on the intraoperative histopathological examination of frozen sections of lymph nodes, segmentectomy including resection of an adjacent segment or subsegment depending on the tumor location (i.e., extended segmentectomy) might thus be established as a standard procedure for curative surgery. Some pure GGO or GGO predominant lesions have no lymph-node metastasis or postoperative recurrence [10]. The evaluation of GGO on high-resolution CT is thus useful for deciding whether limited surgery is indicated. Positron emission tomography (PET) also has an important role in deciding the treatment policy. A larger tumor size and a higher standardized uptake value (SUV) are directly related to the risks of metastasis and invasion. For example, tumors 2 cm or less in diameter with an SUV of ≤1.5 have low risks of metastasis and invasion. Small lung cancers range from low-grade bronchoalveolar cancers presenting with GGO to locally advanced lung cancers consisting primarily of solid components that are accompanied by lymph-node metastasis. Segmentectomy can be indicated for GGO-predominant lesions and predominantly solid lesions 1.5 cm or less in diameter. However, lobectomy is indicated for solid lesions exceeding 1 cm in diameter and should be indicated with caution even for tumors measuring 1 cm or less.
In our department, thoracoscopic limited surgery was intentionally performed in 131 patients with clinical stage IA peripheral lung cancers 2 cm or less in diameter from July 2001 through September 2013 [6–8]. Intentional limited surgery is a procedure designed to reduce the range of resection in patients able to undergo lobectomy. The details of the surgical procedures are shown in Tables 23.1 and 23.2. Single segmentectomy was performed in 63 patients, upper division resection in 15, lingulectomy in 9, extended segmentectomy in 21, single subsegmentectomy in 7, and multiple subsegmentectomy in 16. In segmentectomy with dissection of the pulmonary arteries, veins, and bronchi at the hilum up to the level of the divisions of the segments, the hilar lymph nodes can be evaluated. If lymph-node metastasis is diagnosed on intraoperative histopathological examination of frozen sections of lymph nodes, the surgical procedure is switched to lobectomy. The pros and cons of systematic lymph-node dissection or sampling remain controversial. Systematic lymph-node dissection is recommended for an accurate diagnosis of disease stage. However, the therapeutic effectiveness of this procedure remains a matter of debate. There are certain trends in the most common sites of lymph-node metastasis according to lobe. If no metastasis is found at the most common sites, it has been proposed that selective (rational) lymph-node dissection with omission of more extensive dissection can be performed.
Table 23.1
Segmentectomy
Single segmentectomy | (n = 63) | Extended segmentectomy | (n = 21) |
|---|---|---|---|
Right | Right | ||
S1 | 8 | S1 + S2a | 1 |
S2 | 8 | S1 + S3 | 1 |
S3 | 8 | S1 + S3a | 1 |
S6 | 10 | S2 + S1a | 1 |
S8 | 3 | S6 + S8a | 1 |
Left | S8 + S9 | 2 | |
S1+2 | 7 | S9 + S10 | 3 |
S3 | 3 | Left | |
S4 | 1 | S1+2 + S3a + S3b | 1 |
S6 | 10 | S6 + S9a + S10a | 1 |
S8 | 2 | S8 + S9 | 3 |
S9 | 1 | S9 + S10

Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





