Fig. 35.1
Hyperdense LMCA sign
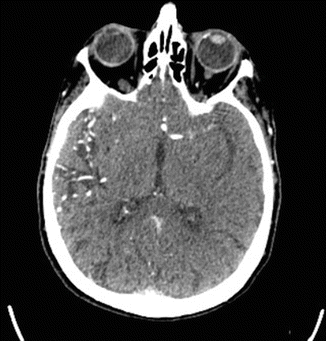
Fig. 35.2
Acute LMCA cut-off
Question
What approach should guide the remainder of this patient’s acute stroke management?
Answer
Emergent endovascular thrombectomy
All patients with acute ischemic stroke (AIS) who present within 6 h of symptoms onset should be evaluated for IV tPA and/or acute endovascular therapy. IV tPA ideally should be dosed within 3 h of symptoms onset, with better outcomes directly correlated to shorter door to thrombolytic times. This patient, following a head CT that revealed no hemorrhage and not having any other contraindication, was started on IV tPA. The stroke and neuro-radiology teams reviewed her imaging and calculated an Alberta Stroke Program Early CT Score (ASPECTS) of greater than 7. She was taken expeditiously to the angiography suite where an NIHSS score by the stroke team was repeated, revealing little to no clinical improvement, and was subsequently started on conscious sedation with midazolam and fentanyl in preparation for endovascular thrombectomy. Neuro-endovascular specialists performed an emergent cerebral diagnostic angiogram (Fig. 35.3), which revealed a persistent left middle cerebral artery occlusion. A stent-retriever device was deployed in the area of occlusion for roughly 2–3 min (Fig. 35.4). Following the single pass attempt, the stent-retriever was pulled with intact clot noted, which was integrated within the stent mesh upon device removal. A confirmatory angiogram was shot immediately following the thrombectomy procedure showing complete recanalization of the left middle cerebral artery with no distal artery cut-off (Fig. 35.5). The patient was then brought to the neurological critical care unit where vital signs and comprehensive neurological assessments were completed every hour for 24 h. Her systolic blood pressure was kept below 180 mmHg and glucose checks were instituted every 4 h with a goal to maintain euglycemia (glucose 80–200 mg/dL). Within a few hours, the patient was able to move her right upper and lower extremity against gravity, her forced gaze deviation was resolved and she was able to follow simple commands. One day later, the patient began speaking more fluently and was able to sit in a chair and tolerate a regular diet. An MRI was completed within 48 h of the endovascular procedure that showed a very small ischemic stroke core on diffusion-weighted imaging and T2 fluid attenuated (FLAIR) imaging. She also underwent an echocardiogram that revealed a 65 % ejection fraction, moderate left atrial enlargement and no apical thrombus. Her CHA2DS2 -VASc was calculated as a 5, and she was started on a regimen of enoxaparin (1 mg/kg) twice daily for secondary stroke prevention as a bridge to the novel anticoagulant apixaban during an outpatient follow-up stroke appointment. The patient continued to make improvements in neurological status and was transferred out of the ICU 2 days after the endovascular therapy with full strength on the right side of her body and only minimal word finding difficulties.
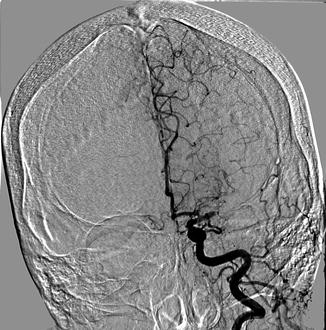
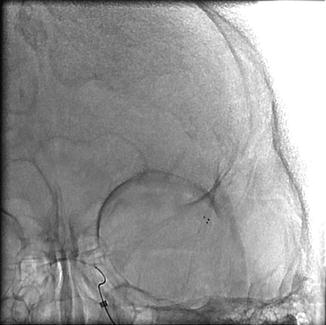
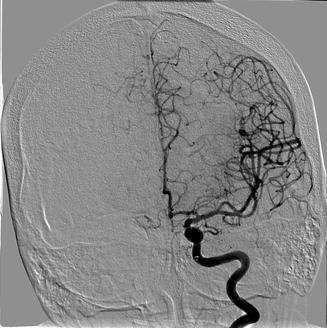

Fig. 35.3
Acute LEFT middle cerebral artery cut-off seen on digital subtraction angiogram

Fig. 35.4
Deployment of stent-retriever into the area of occlusion for roughly 2–3 min

Fig. 35.5
Confirmatory angiogram showing recanalization of the LEFT middle cerebral artery
Principles of Management
Diagnosis
Acute ischemic stroke remains a clinical diagnosis that should be made within the first minutes of a patient entering the emergency department. Tools such as the NIHSS (Table 35.1) help to assess and communicate stroke symptom severity, however in the presence of an acute, focal neurological change, stroke should always be strongly considered. Ideally an initial head CT should be completed as soon as possible and within 25 min of patient arrival to assess for acute intracranial pathology, such as intracranial hemorrhage [1, 2]. If the CT scan is negative for pathology, the patient meets time criteria, does not have a contraindication, and continues to have a neurological deficit suggestive of acute ischemic stroke, IV tPA should be administered [1]. A CT angiogram of the head and neck to assess vessel status, perfusion status and possible vessel occlusion can be completed at the time of the initial CT scan so long as it does not delay the delivery time of IV tPA [1]. An MRI with diffusion-weighted imaging (MRI-DWI) in the stroke patient can be used to show areas of restricted diffusion of water that correspond to areas of acute ischemia. Unlike the head CT for acute stroke imaging, findings on MRI-DWI generally appear within minutes of ischemia.
Table 35.1
NIHSS scoring
1A – Level of consciousness |
Alert; keenly responsive (score 0) |
Arouses to minor stimulation (score 1) |
Requires repeated stimulation to arouse or painful stimulation (score 2) |
Unresponsive, only reflexic posturing (score 3) |
1B – Communication – Ask “What Month is it? How old are you?” |
Both questions correct (score 0) |
Only 1 question answered correctly or if the patients is intubated or has a language barrier (score 1) |
No questions answered correctly/aphasic (score 2) |
1C – Command following – Ask patient to “Blink eyes” and “Squeeze Hands” |
Performs both tasks correctly (score 0) |
Performs one task correctly (score 1) |
Performs none correctly (score 2) |
2 – Horizontal eye movements |
No gaze deviation, palsy (Score 0) |
Partial Gaze Palsy: Can be overcome (Score 1) |
Forced Gaze Palsy: Cannot be overcome, even with oculocephalic reflex (Score 2) |
3 – Visual fields |
No Visual Loss (Score 0) |
Partial Hemianopia (Score 1) |
Complete Hemianopia (Score 2) |
Blind or Bilateral Hemianopia (Score 3) |
4 – Facial weakness |
Normal symmetry (Score 0) |
Minor paralysis, flattened nasolabial fold, smile asymmetry (Score 1) |
Partial paralysis, Lower Face only (Score 2) |
Complete paralysis, upper and lower face (Score 3) |
5a – Left arm: Ask patient to hold LEFT arm up for 10 s |
No drift 10 s, or amputee (score 0) |
Drift, but doesn’t hit the bed (score 1) |
Drift, but it does hit the bed (score 2) |
Is not able to effort against gravity (score 3) |
No movement at all (score 4) |
5B – Right arm: Ask patient to hold RIGHT arm up for 10 s |
No drift 10 s, or amputee (score 0) |
Drift, but doesn’t hit the bed (score 1) |
Drift, but it does hit the bed (score 2) |
Is not able to effort against gravity (score 3) |
No movement at all (score 4) |
6A – Left leg: Ask patient to hold LEFT leg up for 5 s |
No drift for 5 s, or amputee (score 0) |
Drift, but doesn’t hit the bed (score 1) |
Drift, but it does hit the bed (score 2) |
Is not able to lift against gravity (score 3) |
No movement at all (Score 4) |
6B – Right leg: Ask patient to hold LEFT leg up for 5 s |
No drift for 5 s, or amputee (score 0) |
Drift, but doesn’t hit the bed (score 1) |
Drift, but it does hit the bed (score 2) |
Is not able to lift against gravity (score 3) |
No movement at all (score 4) |
7 – Ataxia: finger to nose and heel to shin testing |
No ataxia noted, patient aphasic, patient paralyzed (score 0) |
Ataxia noted in 1 limb (score 1) |
Ataxia noted in 2 limbs (score 2) |
8 – Sensation: testing pain, light touch, vibration sensation bilaterally |
Normal sensation bilaterally (score 0) |
Mild-moderate unilateral loss of sensation (score 1) |
Complete unilateral loss of sensation or unresponsive (score 2) |
9 – Language: testing naming of objects, reading simple sentences, describing a scene |
Normal (score 0) |
Mild – moderate aphasia, decreased ability to communicate but is able to get most ideas out (score 1) |
Severe aphasia – very difficult to communicate, unable to have effective communication (score 2) |
Mute/global aphasia or coma – no speech or auditory comprehension (score 3) |
10 – Dysarthria: repeat or read words testing different parts of the tongue |
Normal speech (score 0) |
Mild-moderate dysarthria – slurred words, but understandable (score 1) |
Mute or severe dysarthria – unable to comprehend speech (score 2) |
11 – Extinction: visual, tactile, auditory, or personal inattention to one side |
No extinction noted (score 0) |
Extinction to one extremity (score 1) |
Profound unilateral extinction, more than one extremity or more than one modality (score 2) |
Score is total out of 41 points |
Acute Stroke Management, tPA Administration
The goal for AIS therapy focuses on revascularization with IV tPA ideally within 45 min of arrival and no later than 1 h after arrival [1, 2]. Once a negative CT result is obtained, the threshold for starting tPA falls to the treating clinician, taking into account the severity of stroke symptoms by NIHSS, the bleed risk of the patient and any known contraindications such as recent major surgery at a non-compressible site, recent myocardial infarction or stroke, or recent or active major gastrointestinal or brain hemorrhage. All patients suspected of AIS above the age of 18 who present within 3 h of known symptoms onset (or last known well time) and have no bleeding or allergic contraindication should receive IV tPA, if feasible to give under that time target [1]. Systemic tPA is dosed at 0.9 mg/kg with 10 % of the dose given as an IV bolus and the remaining 90 % given over the course of an hour [1, 3]. A select group of AIS patients (those not on anticoagulation, younger than 80 years of age and without a previous diagnosis of both stroke and diabetes) continue to have a higher benefit/risk ratio from IV tPA to a 4.5 h time target [1, 4]. Patients taking the novel anticoagulants (Apixaban, Rivaroxiban, Dabigatran, Edoxaban) within the previous 2 days, patients on warfarin with an INR > 1.7 and patients on a therapeutic dose of IV heparin or subcutaneous low-molecular weight heparin should not receive IV tPA [1].
Acute Stroke Management, Endovascular Thrombectomy
Immediately following IV tPA administration, all AIS patients with known large cerebral vessel occlusion per imaging (CTA or MRA) should be considered for endovascular recanalization therapy [1, 5–7]. Several randomized controlled trials published in 2014 and 2015 (i.e. MR CLEAN, SWIFT PRIME, EXTEND IA, ESCAPE, REVASCAT) showed that endovascular therapy is indicated for select patients suffering acute ischemic stroke who present within 6 h of stroke onset (or last known well time) and meet certain imaging and clinical criteria [8–13]. Each of the studies showed a high likelihood of favorable outcome with a number needed to treat of 4 or less. The ideal patients considered for endovascular therapy should have an NIHSS score of greater than 6 and/or a severe deficit such as hemianopsia or aphasia, a CT ASPECTS of greater than 6 [14], a baseline independence of function (modified Rankin score of less than 2 (Table 35.2), show an acute perfusion abnormality to a brain territory, and have no contraindication to contrast-dye administration [6, 13]. Patients who benefited from endovascular therapy in the recent trials were those with a large anterior territory (anterior cerebral artery/middle cerebral artery/carotid terminus) artery occlusion who presented within the first hours of deficit, had an NIHSS score of >6, and were less than 80 years of age (Table 35.1) [8–12]. Patients with posterior territory strokes were not studied. The CT ASPECTS is calculated off of CT source images comparing the stroke-affected side with the unaffected side in 10 separate brain regions [15]. For each region noticeably affected, the ASPECTS is decreased from total score of 10 (no regions affected) to a score of zero (all regions affected).
Table 35.2
Modified Rankin Score (can utilize the mRS-9Q scale [http://www.modifiedrankin.com/] for easy score determination)
Patient has no symptoms of any disease process at all (score 0) |
Patient has mild symptoms of a disease, no disability, is independent (score 1) |
Patient has mild – moderate symptoms of a disease, some disability, is independent (score 2) |
Patient has moderate – severe symptoms of a disease, moderate disability, requires help with some activities of daily living, but is able to walk without assistance (score 3) |
Patient has severe symptoms of a disease, with moderately severe disability and is unable to walk without assistance and is unable to attend to bodily needs without assistance (score 4) |
Patient has severe symptoms of a disease, with severe disability, remains bedridden, incontinent and requires constant nursing care and attention (score 5) |
Patient has died (score 6) |
Supportive Care
All AIS patients should ideally be monitored closely in a dedicated neurosciences ICU or in a stroke unit with continuous monitored telemetry and neurologic expertise for at least the first few days following an event. Patients under 60 years of age with large AIS (greater than 1/3 of a supratentorial hemisphere or a large cerebellar infarct) should be offered decompressive craniectomy within 48 h for definitive malignant cerebral edema management before cerebral herniation occurs and without taking into account which hemisphere (dominant or non-dominant) has infarcted [1]. The DECIMAL, DESTINY and HAMLET studies show strong evidence for an improvement in outcome for patients under 60 years of age with large anterior circulation AIS who undergo early hemicraniectomy [16–18]. Outcomes of “medical optimization” of intracranial pressure with hyperosmolar agents (e.g. mannitol) prior to hemicraniectomy have not been readily studied, and delay of surgery while utilizing these therapies is not recommended [1]. At the time of this manuscript, there are ongoing studies examining whether medications such as IV glyburide for the management of malignant cerebral edema is efficacious in preventing the need for hemicraniectomy [19]. Physical, occupational and speech therapies should be offered to patients as soon as feasible [1]. All AIS patients should undergo a swallowing function examination upon admission and enteral access should be placed if the patient is unable to cooperate [1]. Daily delirium and depression screening and complication management (deep vein thrombosis prophylaxis, secondary pneumonia prevention, urinary tract infection reduction, etc.) is essential for successful outcome [1].
Atrial Fibrillation, Heart Failure and Anticoagulation
Several risk factors for the development of AIS have been identified. Although hypertension, hyperlipidemia, diabetes, vascular diseases and smoking history contribute significantly to one’s stroke risk, atrial fibrillation is one of the most ubiquitous and greatest risk factors for stroke [20–22]. Recently, the CHA2DS2 -VASc scoring system was created and validated to stratify the yearly stroke risk in a patient with atrial fibrillation and compare it to the risk of bleeding from systemic anticoagulation (Table 35.3) [23]. Patients with a score higher than 2 are encouraged to start systemic anticoagulation so long as there are no additional contraindications or increased bleeding risks. Recently, novel anticoagulants (apixaban, edoxaban, dabigatran and rivaroxaban) have been FDA approved for use in patients with non-valvular atrial fibrillation. These novel oral anticoagulants (NOAC) were found to be superior to warfarin for stroke prevention in patients with atrial fibrillation with atrial fibrillation and in some cases (i.e. apixaban) with lower hemorrhage risk [24–27]. Patients with heart failure and an ejection fraction (EF) of <15 % are also at a higher risk for ischemic stroke. The WARCEF trial was completed which compared the efficacy of aspirin to warfarin in the prevention of heart failure related strokes [28, 29]. Among WARCEF patients with heart failure and EF of <15 % who were in sinus rhythm, there was no significant overall difference in the primary outcome between treatment with warfarin and treatment with aspirin.
Table 35.3
CHA2DS2 – VASc Score calculation for atrial fibrillation stroke risk
Congestive Heart Failure (score 1)

Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




