Fig. 27.1
Depiction of usual sites of SMA embolus versus thrombosis. Note sparing of proximal jejunal branches with more distal lodgment of an embolus. Reprinted with permission from Hassoun HT: Acute mesenteric ischemia. Chapter in: Current surgical therapy, 9th ed. Cameron JL (ed.), Mosby, Inc., pp. 884–889
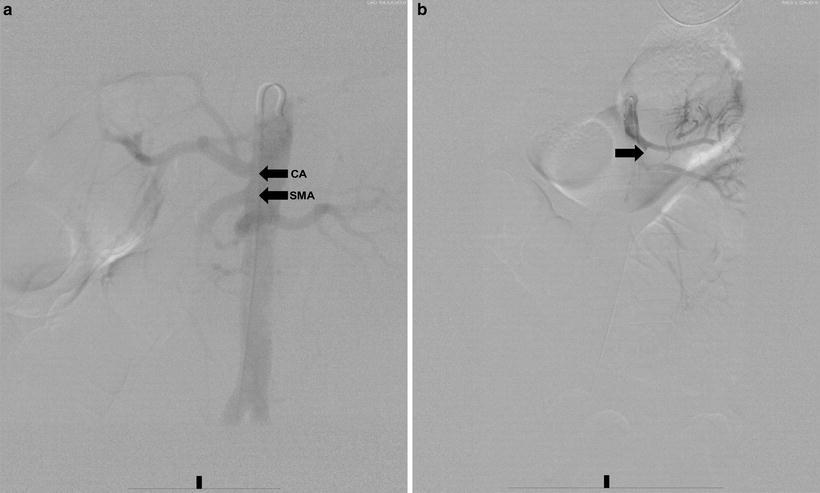
Fig. 27.2
(a) Aortogram demonstrating patent origins of the celiac artery (CA) and SMA. (b) Selective SMA angiogram demonstrating embolic occlusion of the SMA (arrow)
Angiography is less useful for the diagnosis of MVT. Typically, MVT is diagnosed on the venous phase of selective arterial contrast injection; however, conventional angiography is less sensitive and specific for MVT than CTA: the diagnostic imaging modality of choice.
In addition to providing superior imaging quality, contrast angiography enables the surgeon to perform selective injection of any of the mesenteric vessels and to perform therapeutic intervention. In patients with NOMI, for example, the SMA may be selectively catheterized and a vasodilator such as nitroglycerine or papaverine infused directly into the vessel (Fig. 27.3). In a stable patient with AMI from a partially occluding embolus but no peritoneal signs, selective catheterization of the SMA allows the institution of catheter-directed thrombectomy or intra-arterial thrombolytic therapy. Thus, contrast angiography not only represents the gold standard for diagnostic imaging but also provides important therapeutic options.
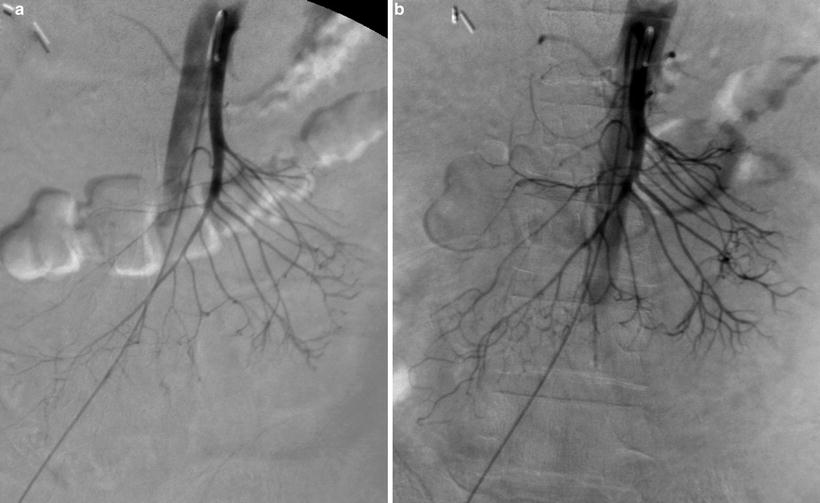

Fig. 27.3
Selective SMA angiogram in a patient with NOMI before (a) and after (b) treatment with catheter-directed papaverine infusion. Note improved filling of more distal SMA branches after treatment
Given the current state of imaging technology, either CTA or MRA can confirm the diagnosis of AMI. Once the cause of ischemia is confirmed, and, in the case of SMA thrombosis, if distal targets are identified for revascularization, it is conceivable that the patient could be explored in the operating room without prior conventional contrast angiography. However, if institutions lack access to a hybrid endovascular suite, formal contrast angiography remains the best imaging modality for evaluation of the mesenteric vasculature.
Management
Embolic Occlusion of Mesenteric Vessels
The goals in surgical treatment of AMI are (1) to restore normal pulsatile flow to the SMA, (2) to resect any nonviable intestine, and (3) to perform second-look laparotomy when viability of the intestine is questionable. In general, revascularization precedes resection. The therapeutic approach varies, depending on the specific underlying cause. For embolic disease of the SMA, the standard treatment is surgical embolectomy.
After initial resuscitation with intravenous (IV) fluids, systemic heparinization, and antibiotics, the patient is taken to the operating room where a midline incision is performed for abdominal exploration. The transverse colon is reflected superiorly and the small bowel is reflected laterally to the patient’s right. The ligament of Treitz is fully incised and the root of the mesentery is fully mobilized. The SMA is easily palpated by placing four fingers of the surgeon’s hand behind the root of the mesentery with the thumb opposite and anterior to the root. The SMA is identified as the firm tubular structure, which may or may not have a palpable pulse. Alternatively, the SMA can also be identified by following the middle colic artery through the transverse colon until it enters the SMA at the root of the mesentery. Proximal and distal control is then obtained by sharp dissection, exposing the artery from its surrounding mesenteric tissue. Patients with SMA embolus will typically have an identifiable pulse proximally in the root of the mesentery with absent pulse distally. Once proximal control is obtained, an arteriotomy (either transverse or longitudinal) is them performed and a Fogarty balloon embolectomy is performed both proximally and distally. The embolus is usually removed with restoration of both back-bleeding as well as return of inflow. The arteriotomy is then closed either primarily or with a patch angioplasty. After restoration of flow, a hand-held continuous wave Doppler can be used to detect the adequacy of intestinal blood flow.
Next, an assessment of bowel viability is performed followed by resection of clearly necrotic or nonviable intestine at this initial exploration. For cases of SMA embolism, the distal small bowel and proximal colon are typically affected with sparing of the proximal jejunum and transverse colon. Determination of bowel viability of marginally perfused intestine can be difficult even in the most experienced hands. Continuous wave Doppler ultrasound of the anti-mesenteric border, intraoperative IV administration of fluorescein and transcutaneous oxygen measurements have all been described, but none of these modalities are sensitive or specific for predicting ultimate bowel viability. Therefore, if any sections of intestine demonstrate questionable viability, the patient should be scheduled for a second-look laparotomy within 24–48 h for resection of nonviable tissue. The decision to perform second-look laparotomy should be made at the initial operation and adhered to strictly; often patients will improve clinically with resuscitation yet will still harbor necrotic bowel that must be removed to prevent systemic sepsis.
Percutaneous interventional treatment of the SMA occlusion has been described in the literature. At present, however, the applicability of this approach is limited, since most patients present with symptoms that warrant an exploratory laparotomy for evaluation of intestinal viability. In patients who present with abdominal pain and have no peritoneal signs that would necessitate immediate laparotomy, catheter-directed intra-arterial thrombolytic therapy of partially occlusive SMA emboli can be considered. Case reports have documented successful thrombolytic therapy, angioplasty and stenting in patients with AMI [21]; however, this route should be used cautiously and in the correct patient population (i.e., those without peritoneal signs or radiographic suggestion of bowel infarction). These patients will require close monitoring in the intensive care unit (ICU) setting with frequent abdominal examinations, and even if catheter-directed therapy does restore flow to affected bowel, the patient may still experience pain sufficient to warrant exploration. For these reasons, our use of thrombolytic therapy is highly selective.
SMA Thrombosis
AMI secondary to acute SMA thrombosis occurs in patients with long-standing atherosclerotic disease of the mesenteric vessels, and the entire midgut is usually involved. Surgical treatment consists of a bypass procedure, which may be done in either an anterograde or retrograde manner. The decision regarding the optimal method is often made intraoperatively based on the quality of the inflow vessels and patient condition. The conduit of choice is a reversed autologous greater saphenous vein graft. If possible, synthetic graft material should be avoided in the setting of acute bowel ischemia, given the risk of transmural infarction and bowel perforation. There are several inflow options for revascularization of the SMA including the supraceliac aorta, the infra-renal aorta, and the iliac arteries. While antegrade bypass graft of the supraceliac aorta to the SMA tunneled behind the pancreas is the optimal configuration because of less susceptibility to kinking, retrograde bypass from either the infra-renal aorta or iliac artery may be easier to perform in the acute setting when rapid revascularization is the ultimate goal. Additionally, retrograde bypass results in less hemodynamic compromise by avoiding supraceliac clamping and associated mesenteric and renal ischemia. Many of these patients, however, will have severe atherosclerotic disease precluding retrograde bypass and therefore the surgeon should be ready to perform revascularization from either approach.
Recently, a combined open and endovascular approach has been described [22]. With this technique, the infracolic SMA is exposed as usual and following thrombectomy and patch angioplasty, a sheath is placed in the infracolic SMA through the distal end of the patch for retrograde cannulation and stenting of the lesion. This hybrid technique offers both the advantages of open laparotomy for assessment of bowel viability and endovascular management for rapid revascularization, thus limiting ischemic time.
Patients with severe comorbidities without signs of peritonitis who present with acute SMA thrombosis may occasionally be treated with catheter-directed thrombolysis followed by percutaneous angioplasty and stenting; however, this treatment modality should be performed selectively and patients should be monitored closely for the need to undergo surgical exploration.
Non-occlusive Mesenteric Ischemia
Management of NOMI is largely nonoperative, and once the diagnosis has been established with angiography, treatment of the underlying precipitating cause is the key therapeutic intervention. Fluid resuscitation, optimization of cardiac output, and elimination of vasopressors are primary measures that greatly impact outcome. Selective SMA catheterization and papaverine infusion (30–60 mg/h) offers adjunctive therapy, and the infusion is continued for 24–48 h with repeat angiography at regular intervals to gauge efficacy. This algorithm is reserved for patients with hemodynamic stability and no signs of peritonitis on physical examination. Alternative therapy has been described using intra-arterial tolazoline and glycerol trinitrite as local dilators with good success [23].
If a patient presents with peritoneal signs, an exploratory laparotomy will be required for resection of frankly necrotic or gangrenous bowel. Intra-arterial papaverine infusion started prior to operation can be continued throughout surgical exploration. Additionally, given the propensity of NOMI to wax and wane in severity, a second-look laparotomy becomes imperative (see later section: Second-Look Laparotomy).
Mesenteric Venous Thrombosis
The mainstay of therapy for MVT is anticoagulation; however, if the patient’s condition does not improve or worsens or if signs or symptoms of bowel ischemia develop, abdominal exploration is warranted. Most patients with MVT can be successfully managed with anticoagulation alone [8]; however, many will still require small bowel resection. Thrombolytic therapy can also treat MVT, with the catheter being placed into either the SMA for lysis of portal vein thrombus [8] or into the SMV or portal vein intraoperatively [24].
Additionally, once the diagnosis of MVT has been established, a hypercoagulable workup should be initiated to identify the underlying cause. If the patient has a hematologic hypercoagulable state, lifelong anticoagulation is recommended; however, if the cause is reversible, anticoagulation can be discontinued after 3–6 months.
Second-Look Laparotomy
Second-look laparotomy is an essential part of AMI management. Regardless of which adjunctive measure is employed intraoperatively to assess bowel perfusion and viability, second-look laparotomy is the most reliable means of determining the viability of marginally perfused bowel after revascularization. Indications for a second look include presentation with a low-flow state, requirement for small bowel resection and anastomosis, or requirement of a mesenteric thromboembolectomy [25]. Prior to a second look, appropriate fluid resuscitation and correction of any metabolic imbalances should be undertaken. Furthermore, the decision to return to the operating room for a second look should be made upon initial exploration, and should not be foregone regardless of the patient’s condition 24–48 h later. Often, patients may retain necrotic bowel even after correction of metabolic derangements and volume status.
Some authors have advocated the use of second-look laparoscopy as an alternative to repeat laparotomy, citing lower operative times, shortened anesthesia requirements, and fewer postoperative complications such as wound complications [26]. The role of laparoscopic second-look operations remains unknown; however, an increasing number of recent publications reflect the widening experience with this modality [25, 27].
Anatomic Considerations
The splanchnic vasculature follows a well-described pattern with commonly identified variations that are crucial to understanding the presentation and pathogenesis of AMI. Important variations from classic splanchnic arterial anatomy include a common celiacomesenteric trunk, “replaced” hepatic arterial branches from the superior mesenteric artery (SMA) supply as opposed to their usual celiac origin, and the “Arch of Buhler”: persistent ventral anastomosis between the proper hepatic and the replaced right hepatic from the SMA [28].
The SMA arises from the abdominal aorta 1–2 cm below the origin of the celiac trunk. Classically, the hepatic arteries arise from the celiac axis via the common and proper hepatic arteries; however, the right hepatic artery obtains its origin from the SMA in 15–20% of patients and the left hepatic artery branches from the left gastric artery in 25% of patients [29]. Should the celiac or superior mesenteric arteries experience an acute occlusion, the gastroduodenal artery becomes an important source for collateral flow. Additional SMA vascular anastomotic arcades occur with varying degrees of development among patients, with important implications during AMI. Large-vessel anastomoses arise along the 10–20 jejunal and ileal branches from the SMA. An anastomosis between the SMA and inferior mesenteric artery (IMA) occurs between the middle and left colic branches of the SMA and the IMA at the splenic flexure of the colon, termed “Griffith’s point,” a watershed area. The IMA arises from the abdominal aorta 5–6 cm below the origin of the SMA, supplying the left half of the transverse colon and the descending colon via the left colic artery. The marginal artery of Drummond and the arc of Riolan are important SMA and IMA collaterals that are capable of enlarging upon occlusion of the proximal splanchnic arteries.
The venous anatomy of the splanchnic system parallels the arterial anatomy, and the confluence of the superior mesenteric and splenic veins forms the portal vein, supplying vital perfusion to the liver. Hepatic blood then drains into the systemic circulation via the right, left, and middle hepatic veins into the superior vena cava. Specific sites of porto-systemic collateral circulation are of great importance during portal hypertension, which is beyond the scope of this chapter. However, in the event of MVT, these collaterals may become enlarged similar to the pattern seen in patients with portal hypertension.
Intestinal blood flow comprises 10–20% of the cardiac output, with significant increases in SMA, but not celiac, flow occurring 20–30 min after meal ingestion and sustain for 90 min. The intestinal mucosa comprises 1/2 of intestinal mass; however, it receives 75% of resting intestinal blood flow, with the remainder supplying the muscular and serosal layers. The sympathetic nervous system serves as the primary regulator of splanchnic blood flow, with influences from metabolic, myogenic, and extrinsic factors. Sympathetic stimulation increases splanchnic vascular tone, decreasing blood flow. Numerous hormonal and molecular substances contribute to the regulation of splanchnic blood flow in addition to many pharmaceuticals, some which may contribute to AMI in states of low systemic blood pressure.
Potential Complications
Mesenteric Ischemia and Reperfusion
Although AMI is initially managed surgically, patients face a significant risk of morbidity and mortality after treatment from systemic inflammation and subsequent multiple organ dysfunction syndrome (MODS). Mesenteric ischemia–reperfusion injury (IRI) promotes local synthesis of inflammatory mediators that exacerbate gut injury, priming circulating neutrophils for enhanced superoxide anion production and subsequent remote (i.e., pulmonary, hepatic) injury [30]. At the cellular level, mesenteric IRI activates a cascade of oxidative stress-sensitive protein kinases that converge on specific transcriptional factors to regulate expression of pro-inflammatory genes. These gene targets include enzymes (inducible nitric oxide synthase [iNOS] cyclooxygenase, and phospholipase A2), cytokines (tumor necrosis factor-α [TNF-α] and interleukin [IL]-1), chemokines (IL-8), and adhesion molecules (intercellular adhesion molecule-1 [ICAM-1]) [31–36]. Excessive gene activation leads to a maladaptive systemic inflammatory response syndrome (SIRS) that can trigger early MODS. Locally, this hyperinflammatory state can cause gut dysfunction characterized by histologic evidence of mucosal injury, increased intestinal epithelial and microvascular permeability, and impaired motility. Patients then become more susceptible to bacteremia, endotoxemia, and eventually, late MODS.

Full access? Get Clinical Tree







