58 Acute Lung Injury and Acute Respiratory Distress Syndrome
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are common problems in the intensive care unit (ICU) and can complicate a wide spectrum of critical illnesses. First described by Ashbaugh in 1967,1 the syndrome was initially termed adult respiratory distress syndrome to distinguish it from the respiratory distress syndrome of neonates. However, with the recognition that ALI/ARDS can occur in children, the term acute has replaced adult in the nomenclature in recognition of the typical acute onset that defines the syndrome. Although specific treatments for ALI/ARDS have been slow to emerge, the recent development of new strategies for mechanical ventilation that improve mortality, and fluid management strategies that reduce the length of mechanical ventilation, emphasizes the importance of identifying and appropriately treating all patients with ALI/ARDS. Although this point would seem to be straightforward, in practice, ALI/ARDS remains largely underdiagnosed,2,3 and often expert practitioners disagree on the diagnosis,4 which perpetuates inappropriate or inadequate treatment.
 Epidemiology
Epidemiology
The exact incidence of ALI/ARDS has been difficult to estimate for a variety of reasons. In the past, variable definitions of the syndrome were used.5 The wide variety of causes and coexisting disease processes has also made identification of cases difficult, both at the clinical and administrative coding level.6 The National Institutes of Health first estimated the incidence at 75 per 100,000 population in 1977,7 but a number of studies since then have reported lower incidences.6 Two prospective studies confirmed the higher original National Institutes of Health Estimate. The first utilized enrollment logs from the National Heart, Lung and Blood Institute–sponsored ARDS Network of 20 hospitals and estimated that the incidence could be as high as 64 cases per 100,000 population. This dataset has the advantage of being prospectively collected from a large number of academic medical centers. The second was a large prospective study of residents of King County, Washington. In that study, the crude incidence of ALI/ARDS in adults was 78.9 per 100,000 patient years.8 A large prospective European study of the incidence of ARDS found that ALI occurred in 7.1% of all hospital admissions.9 A third of these patients presented with only mild ALI, but of these, half progressed rapidly to ARDS. Some studies suggest a decline in the incidence of ARDS over time. A large prospective cohort of trauma patients at risk for ARDS and multisystem organ failure collected over time showed that the incidence of ARDS decreased from 43% in 1997 to 12% in 2004, a finding that may reflect advances in posttrauma critical care.10 Regardless of the exact incidence, it is clear that ALI/ARDS is a major public health problem that will be encountered frequently by all physicians who care for critically ill patients.
 Risk Factors
Risk Factors
ALI/ARDS can occur as a result of either direct or indirect injury to the lungs (Table 58-1) in patients with a predisposing risk factor. The commonly associated clinical disorders can be separated into those that directly injure the lung and those that indirectly injure the lung. Although it is not always feasible to determine the exact cause of ALI/ARDS in a given patient, direct causes appear to account for approximately half of all cases of ALI/ARDS.11 It is not clear whether the distinction between direct and indirect lung injury is clinically useful.12 Some investigators have demonstrated reduced respiratory system compliance in patients with ARDS due to direct pulmonary injury compared to indirect causes,13 although total respiratory system compliance (including the chest wall) is similar.14 Patients with direct lung injury may be more likely to have improved lung mechanics with the application of PEEP. However, in the largest cohort of patients studied to date, there was no difference in mortality between those with direct (pulmonary) and indirect (extrapulmonary) causes of lung injury.11 Regardless of the underlying cause of ALI/ARDS, most patients with ALI/ARDS appear to have a systemic illness with inflammation and organ dysfunction not confined to the lung.15
TABLE 58-1 Risk Factors Associated with Development of Acute Lung Injury and Acute Respiratory Distress Syndrome
| Direct Lung Injury | Indirect Lung Injury |
|---|---|
| Pneumonia | Sepsis |
| Aspiration of gastric contents | Multiple trauma |
| Pulmonary contusion | Cardiopulmonary bypass |
| Fat, amniotic fluid, or air emboli | Drug overdose |
| Near-drowning | Acute pancreatitis |
| Inhalational injury | Transfusion of blood products |
| Reperfusion pulmonary edema |
Sepsis is the most common cause of indirect lung injury, with an overall risk of progression to ALI or ARDS of approximately 30% to 40%.16–19 In a more recent prospective study of hospitalized patients with a risk factor for acute lung injury (e.g., sepsis, pneumonia) 6.5% of patients developed ALI, and 4% met criteria for ARDS; the risk was higher with multiple risk factors.20 In addition to sepsis itself being a risk factor for development of ARDS, the site of infection may also influence the risk of lung injury. In patients with sepsis admitted to an ICU, patients who had pneumonia as the source of sepsis had an increased risk of ARDS compared to those with infections at other sites such as the abdomen, skin, or soft tissue.21 Severe trauma with shock and multiple transfusions also can cause indirect lung injury. Although the other causes of indirect lung injury are less common, many, such as blood transfusions, are commonplace events in the ICU setting. The most common cause of direct lung injury is pneumonia, which may be of bacterial, viral, or fungal origin. The risk of developing ALI/ARDS increases substantially in the presence of multiple predisposing disorders.19 Secondary factors may also increase the risk. Such factors include chronic lung disease,18 chronic or acute alcohol abuse,22,23 increasing age,24 transfusion of blood products,25–27 lung resection,28 and obesity.24 Emerging evidence has suggested that some at-risk patients may actually be protected from the development of ARDS. Several studies have shown that patients with diabetes are less likely to develop ARDS.29–31 To some extent, every patient in the ICU is at risk for developing ALI/ARDS, and vigilance is required to recognize the diagnosis and treat appropriately.
 Pathophysiology
Pathophysiology
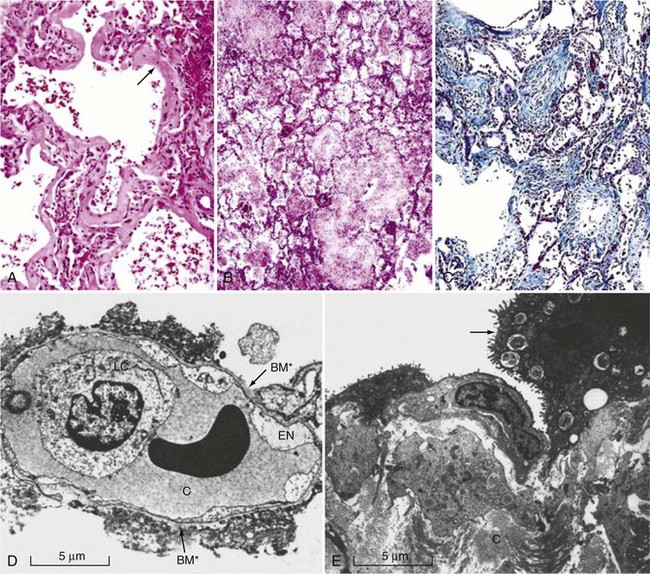
The pathophysiology of ALI/ARDS is complex and remains incompletely understood. Microscopically, lungs from afflicted individuals in the early stages show diffuse alveolar damage with alveolar flooding by proteinaceous fluid, neutrophil influx into the alveolar space, loss of alveolar epithelial cells, deposition of hyaline membranes on the denuded basement membrane, and formation of microthrombi (Figure 58-1).32 Alveolar flooding occurs as a result of injury to the alveolar-capillary barrier and is a major determinant of the hypoxemia and altered lung mechanics that characterize early ALI/ARDS.
The alveolar-capillary barrier is formed of two separate cell layers, the microvascular endothelium and the alveolar epithelium. Injury to the alveolar epithelium is a prominent histologic feature, with loss of alveolar epithelial barrier integrity and sloughing of alveolar epithelial type I cells. Alveolar epithelial apoptosis is widespread and likely contributes to the loss of epithelium seen ultrastructurally.33,34 Although endothelial injury is less obvious at the microscopic level, ultrastructural studies reveal that it is widespread.35,36 Endothelial injury allows leakage of plasma from the capillaries into the interstitium and airspaces. Alveolar flooding in ALI/ARDS is characteristically with a protein-rich edema fluid, owing to the increased permeability of the alveolar capillary barrier, in contrast to the low-protein pulmonary edema that results from hydrostatic causes such as congestive heart failure or acute myocardial infarction.37–40
Neutrophils play an important role41 in the initial inflammatory response in ARDS. Early ALI/ARDS is characterized by migration of neutrophils into the alveolar compartment.35,36 Neutrophils can release a variety of injurious substances, including proteases such as neutrophil elastase, collagenase, and gelatinases A and B, as well as reactive nitrogen and oxygen species. In addition, they can elaborate proinflammatory cytokines and chemokines which amplify the inflammatory response in the lung. Resident alveolar macrophages are also involved in initiating and sustaining a proinflammatory cytokine cascade that leads to recruitment of neutrophils into the lung.
In addition to acute neutrophilic inflammation and elaboration of a proinflammatory cytokine cascade, a variety of other abnormalities contribute to the pathogenesis of ALI/ARDS. Surfactant dysfunction is characteristic, with abnormalities in both the protein and lipid components.42–45 This likely results from disruption of normal surfactant activity secondary to the influx of plasma proteins into the airspaces, intraalveolar proteolysis, and injury to the alveolar epithelial type II cells. Surfactant dysfunction may have important implications both for lung mechanics and host defense.46 Activation of the coagulation cascade and impaired fibrinolysis are also apparent in patients with ALI/ARDS,47,48 both in the lung49–51 and systemically.52,53 Alteration in the balance of endogenous oxidants and antioxidants, with a decrease in endogenous antioxidants54 despite the increased oxidant production, has also been observed.55
The contribution of ventilator-associated lung injury to the pathogenesis of ALI/ARDS has been recognized. There are several mechanisms by which mechanical ventilation can injure the lung. Ventilation at very high volumes and pressures can injure even the normal lung, leading to increased permeability pulmonary edema due to capillary stress failure56 and sustained elevations of circulating plasma cytokines.57 In the injured lung, even tidal volumes that are well tolerated in the normal lung can lead to alveolar overdistension in relatively uninjured areas because the lung available for distribution of the administered tidal volume is greatly reduced and because of uneven distribution of inspired gas.58,59 In addition to alveolar overdistension, cyclic opening and closing of atelectatic alveoli can cause lung injury even in the absence of alveolar overdistension. The combination of alveolar overdistension with cyclic opening and closing of alveoli is particularly harmful and can initiate a proinflammatory cascade.60
A ventilatory strategy that was designed to minimize alveolar overdistension and maximize alveolar recruitment ameliorated proinflammatory cytokine release.61 This fundamental insight into the pathogenesis of clinical ALI/ARDS has led to multiple clinical trials of novel ventilatory strategies for patients with ALI/ARDS, including the landmark ARDS Network trial of 6 mL/kg versus 12 mL/kg tidal volume ventilation62 (see Treatment section).
 Diagnosis
Diagnosis
In 1994, the American-European Consensus Conference published new clinical definitions for ALI and ARDS.5 Prior to this time, a variety of definitions were used clinically, including the Murray Lung Injury Score.63 To meet the Consensus diagnostic criteria for either ALI/ARDS, the acute onset of bilateral radiographic infiltrates is required. There should be no clinical evidence of left atrial hypertension, with a pulmonary artery occlusion pressure (PAOP) ≤ 18 mm Hg if measured. Although not strictly part of these definitions, an underlying cause of lung injury should be sought. In the absence of an identifiable underlying cause (see Table 58-1), particular attention should be given to the possibility of other causes of pulmonary infiltrates and hypoxemia, such as hydrostatic pulmonary edema. One potential limitation of the consensus definition is the need for arterial blood gas sampling to calculate a PaO2/FIO2 ratio. Recent work has shown good correlation between the SpO2/FIO2 ratio (measured by pulse oximetry) and the PaO2/FIO2 ratio,64,65 with an SpO2/FIO2 ratio of 235 corresponding to a PaO2/FIO2 ratio of 200, and an SpO2/FIO2 ratio of 315 correlating to a PaO2/FIO2 ratio of 300. These calculations are valid only when the SpO2 is less than 98%, because the oxyhemoglobin dissociation curve is flat above this level. Oxygen saturation is a noninvasive, continuously available measurement; use of the SpO2/FIO2 ratio may improve the ability of clinicians to diagnose ARDS.
Differentiating ARDS from hydrostatic edema can be difficult, and there may be significant overlap in the syndromes (Figure 58-2).66 A recent multicenter trial on intravenous catheter directed fluid management strategies in patients with ARDS showed that 29% of patients with clinically defined ARDS had a PAOP greater than 18 mm Hg at the time of pulmonary artery catheter insertion, but that 97% of patients had a normal or elevated cardiac index, suggesting they did not have clinical heart failure.67 Other studies have shown similar rates of elevated PAOP in patients with ARDS.68
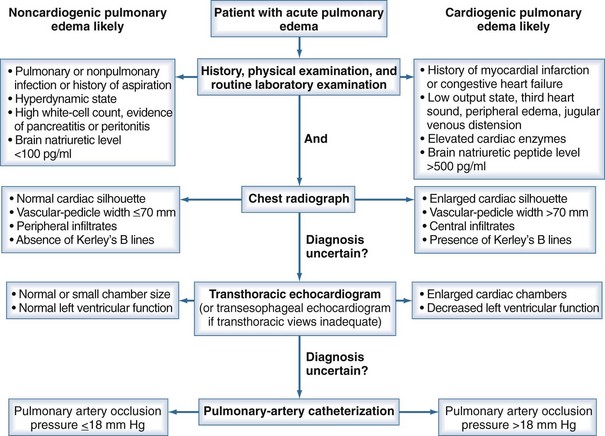
Figure 58-2 Algorithm for differentiating between cardiogenic and noncardiogenic pulmonary edema.
(With permission from Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005;353[26]:2788-2796.)
There are no specific clinical or laboratory studies that can reliably distinguish between ARDS and hydrostatic edema. A study examining the diagnostic utility of serum levels of B-type natriuretic peptide (BNP) showed that BNP measured at admission could not reliably differentiate between hydrostatic edema and ARDS. Furthermore, BNP levels in these patients did not correlate with invasive hemodynamic measurements.69
The standardization of definitions for ALI and ARDS has been helpful from several perspectives. For clinical research, it has been valuable in allowing the comparison of different studies and the rapid identification of patients for enrollment in clinical trials. Clinically, the new definitions are easy to apply and facilitate rapid identification and appropriate treatment of patients with ALI/ARDS. However, it should be noted the nature of ALI/ARDS is such that any definition will have significant shortcomings. First, the definitions must be based solely on clinical criteria, because currently there is no laboratory test that allows clinical assessment of the presence or absence of ALI/ARDS. Second, there is no reference to pathogenesis or underlying cause. This is because the list of potential causes of ALI/ARDS is so long, diverse, and common in the critically ill. Third, the presence or absence of multiorgan dysfunction, an important determinant of outcome, is not specified. Finally, though the presence of bilateral infiltrates has major prognostic significance and is clearly a hallmark of the syndrome, the radiographic findings are not specific for ALI/ARDS.4,70 Diagnostic uncertainty in ALI/ARDS is a major barrier to initiation of appropriate therapy and one of the main reasons why clinicians fail to initiate lung-protective ventilation in clinically appropriate patients.71
Recent work has focused on alternative methods to increase sensitivity and specificity of the clinical definitions for ALI/ARDS. The pulmonary edema fluid–to–plasma protein ratio can reliably distinguish between low-permeability (hydrostatic edema) and high-permeability (ARDS) pulmonary edema if measured early after endotracheal intubation,72 but prospective validation is still needed. Alternatively, circulating biomarkers may prove useful for the diagnosis of ALI/ARDS.73 Despite its shortcomings, the current clinical definition of ALI/ARDS based on consensus criteria should be used to rapidly identify patients with ALI/ARDS so appropriate therapy can be initiated promptly.
In the majority of patients, the initial diagnosis of ALI/ARDS is made clinically. Invasive techniques for diagnosis are of limited clinical utility, and the benefits rarely outweigh the risks. Bronchoscopy may be indicated in the early phases of ALI/ARDS in patients in whom there is no identifiable predisposing risk factor. Rarely, an alternate treatable diagnosis is found, such as acute eosinophilic pneumonia, pulmonary alveolar proteinosis, diffuse alveolar hemorrhage, or unsuspected infection. Bronchoalveolar lavage for cultures and cytologic examination can identify the cause of pneumonia, and is particularly useful in the diagnosis of opportunistic infections. Lavage fluid usually has a predominance of neutrophils, and there may be evidence of diffuse alveolar hemorrhage. Cytologic examination can be used to confirm the presence of diffuse alveolar damage.74
In the past, open lung biopsy was obtained more frequently for diagnosis in patients with suspected ARDS. Interestingly, the degree of histologic abnormality on lung biopsy does not correlate with ultimate outcome as measured by pulmonary function.75 Open or thoracoscopic lung biopsy may still be useful in some cases where the diagnosis is uncertain and the underlying cause is not apparent. Although open lung biopsy can provide findings that lead to a change in treatment, postoperative complications can occur in 20% of patients.76 Several pathologic studies have shown that biopsy or autopsy can identify unsuspected diagnoses requiring specific therapy (e.g., miliary tuberculosis, pulmonary blastomycosis, aspergillosis, bronchiolitis obliterans organizing pneumonia) in 40% to 60% of cases,76–78 although the general applicability of these studies may be limited by the fact that they were retrospective case series.
 Clinical Course
Clinical Course
Early Ali/Ards
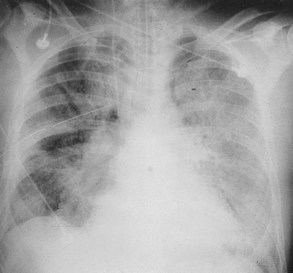
The Consensus definitions are designed to identify ALI/ARDS patients early in their course, in the acute or exudative phase. Clinically, the acute phase is manifested by the acute onset of radiographic infiltrates consistent with pulmonary edema, hypoxemia, and increased work of breathing. Radiographic infiltrates are bilateral (by definition), but may be patchy or diffuse, fluffy or dense (Figure 58-3), and pleural effusions may occur.79 Chest computed tomographic (CT) imaging, though rarely of use clinically, has been employed extensively as an investigative tool to better define the nature of the infiltrates in patients with ALI/ARDS. The distribution of infiltrates by CT is surprisingly patchy; areas of alveolar filling and consolidation occur predominantly in dependent zones, while non-dependent regions can appear relatively spared.80–82 Even areas that appear spared in conventional radiographic images may have substantial inflammation when sampled using bronchoalveolar lavage83 or using FDG-PET scanning.84
The hypoxemia that characterizes early ALI/ARDS is usually relatively refractory to supplemental oxygen. The increased work of breathing in the acute phase of ALI/ARDS is due to decreased lung compliance as a result of alveolar and interstitial edema combined with increased airflow resistance85 and increased respiratory drive.86 The combination of hypoxemia and increased work of breathing usually necessitates endotracheal intubation and mechanical ventilation, although occasionally patients can be managed with noninvasive ventilation (see Treatment section).
In addition to hypoxemia and increased work of breathing, many patients with ARDS also develop evidence of increased pulmonary vascular resistance leading to pulmonary hypertension and RV failure. The prevalence of pulmonary hypertension in patients presenting to the hospital with ARDS may be as high as 92%,87 and as many as 10% of patients with ARDS may have right ventricular (RV) failure defined by hemodynamic measurements.88 Nevertheless, the presence of RV failure does not impact mortality. Attempts to reverse pulmonary hypertension and RV failure with pulmonary vasodilators such as sildenafil have led to decreased pulmonary artery pressure with treatment, as well as concomitant increases in shunt fraction and decreases in oxygenation.89 These findings suggest that although patients with ARDS have evidence of pulmonary hypertension, it may in some cases be a beneficial physiologic response to reduce blood flow to areas of severely compromised lung.
Late Fibroproliferative Ali/Ards
In most patients, ALI/ARDS will substantially resolve after the acute phase. However, in others, the syndrome progresses to a fibrosing alveolitis. Fibrosing alveolitis usually becomes clinically apparent after 7 to 10 days, although evidence of deposition of extracellular matrix has been identified in alveolar lining fluid from patients as early as the first day after intubation.90 Radiographically, linear opacities develop, consistent with the evolving fibrosis. Histologically, pulmonary edema and neutrophilic inflammation are less prominent. A severe fibroproliferative process fills the airspaces with granulation tissue that contains extracellular matrix rich in collagen and fibrin, as well as new blood vessels and proliferating mesenchymal cells.91,92
Clinically, the late fibroproliferative phase of ALI/ARDS is characterized by continued need for mechanical ventilation, often with persistently high levels of PEEP and FIO2. Lung compliance may fall even further, and pulmonary dead space is elevated. If it has not developed in the acute phase, pulmonary hypertension may occur now owing to obliteration of the pulmonary capillary bed, and right ventricular failure may appear.93 This phase of the illness can be prolonged, lasting weeks, and can be very frustrating for the clinician, patient, and family; small gains in pulmonary function are frequently offset by new problems such as hospital-acquired infections, organ failures, or barotrauma. Progressive deconditioning can make eventual weaning from mechanical ventilation difficult if the fibrosing alveolitis stage is prolonged. Based on improvement in number of ventilator-free days through use of lower tidal volumes, it seems likely the incidence of fibrosing alveolitis will fall.
Resolution of Ali/Ards
Lung biopsies from ALI/ARDS survivors typically show normal or near-normal lung histology. For such histologically complete resolution of ALI/ARDS to occur, a variety of processes must be reversed. Alveolar edema is actively reabsorbed by the vectorial transport of sodium and chloride from the distal airway and alveolar spaces into the lung interstitium.94 Water is passively absorbed along the osmotic gradient, probably through water channels, the aquaporins.95 The majority of patients with early ALI/ARDS have impaired alveolar fluid transport, but in those with intact alveolar fluid transport, faster rates of alveolar epithelial fluid transport are associated with better outcomes.37 Soluble and insoluble protein must also be cleared from the airspaces. Soluble protein probably diffuses by a paracellular route into the interstitium, where it is cleared by lymphatics. Insoluble protein probably is cleared by macrophage phagocytosis or alveolar epithelial cell endocytosis and transcytosis.96
The denuded alveolar epithelium in ALI/ARDS must be repaired. The alveolar epithelial type II cell serves as the progenitor cell for repopulating the alveolar epithelium. Type II cells proliferate, migrate, and differentiate to reconstitute a tight alveolar epithelial type I cell barrier. The inflammatory cell infiltrate must also resolve, but here the mechanisms are less clear. Resolution of neutrophilic inflammation may be predominantly via neutrophil apoptosis and phagocytosis by macrophages. However, one report suggests that neutrophil apoptosis is impaired in the lungs of patients with ALI/ARDS.97 The resolution of fibrotic changes is also not well understood. Clearly, however, substantial remodeling is necessary to restore a normal or near-normal alveolar architecture. In patients with advanced fibrosis, this process likely takes place over many months; pulmonary function abnormalities continue to improve, sometimes remarkably so, out to the first year in survivors of ALI/ARDS (see later discussion).98
 Treatment
Treatment
Standard Supportive Therapy
Treatment of Predisposing Factors
First and foremost, a search for the underlying cause of ALI/ARDS should be undertaken. Appropriate treatment for any precipitating infection such as pneumonia is critical to enhance the chance of survival. In the immunocompromised host or patients without predisposing risk factors, invasive diagnostic evaluation including bronchoscopy may be warranted to look for evidence of opportunistic infections or alternative specific causes of ARDS. In a patient with sepsis and ALI/ARDS of unknown source, an intraabdominal process should be considered. Timely surgical management of intraabdominal sepsis is associated with better outcomes.99 In some patients, the cause of lung injury will not be specifically treatable (such as aspiration of gastric contents) or will not be readily identifiable.
Fluid and Hemodynamic Management
There are data supporting the use of early goal-directed therapy to support cardiac output and oxygen delivery within a set range with fluids, inotropes, and blood transfusions using central venous oxygen saturation as a therapeutic driver in patients who have severe sepsis and septic shock,100 many of whom develop ALI/ARDS. But this approach has not been specifically studied in ALI/ARDS. Historically, patients with critical illness and ALI/ARDS received a pulmonary artery catheter (PAC) to manage fluid and hemodynamic status. A large, randomized European trial of PAC use versus no PAC use in all patients admitted with ARDS101 showed no difference in clinical outcomes in either group, suggesting that routine PAC use in ARDS is not beneficial. The ARDS Clinical Trials Network tested the value of pulmonary artery catheterization in the context of specific fluid-management protocols and was unable to demonstrate improved outcomes through use of the PAC.67 Some investigators have proposed that clinical outcomes in ALI/ARDS can be improved by delivery of supranormal levels of oxygen to the tissues using vigorous volume resuscitation and positive inotropes. However, no benefit to supranormal levels of oxygen delivery has been demonstrated in patients with ALI/ARDS.102,103
For decades there was disagreement as to the best fluid-management strategy in patients with ARDS. Proponents of a liberal fluid strategy reasoned that increased circulating volume would preserve end-organ perfusion and protect patients from the development of non-pulmonary organ failures. Reductions in intravascular volume can have adverse effects on cardiac output and tissue perfusion, factors that could contribute to multisystem organ failure. This is a legitimate concern, since mortality in ALI/ARDS is usually from non-pulmonary causes including other organ failures. Others supported a conservative fluid strategy in an attempt to reduce circulating volume, thereby reducing the driving force for pulmonary edema formation. In experimental lung injury, lower left atrial pressures are associated with less formation of pulmonary edema.93,104 There is some clinical evidence to support this approach.105–108 Given the equipoise with the approach to fluids in ALI/ARDS, the ARDS Network conducted a large, multicenter, randomized controlled trial of catheter-driven (central venous catheter versus PAC) fluid management in patients with ALI.109 Once patients were out of shock, they were randomized to a liberal fluid treatment strategy that resulted in an average of 1 liter of fluid accumulation per day or to a conservative fluid treatment strategy with aggressive use of diuretics to achieve a goal central venous pressure (CVP) below 4 or a goal PAOP below 8, an approach that resulted in an average of zero net fluid accumulation by day 7. Although there was no difference in mortality at 60 days (the primary outcome of the study), patients in the conservative group had improved oxygenation and significantly more ventilator-free days without the development of additional organ failures. In this study, it did not matter whether treatment was guided by CVP measurements (derived from a central venous line) or from PAOP measurements (derived from a PAC).110
Despite the findings in support of conservative fluid management strategy in patients with ARDS, there continues to be a great deal of uncertainty about appropriate goals for hemodynamic therapy in ALI/ARDS. Currently, the recommended strategy is to aim to achieve the lowest intravascular volume that maintains adequate tissue perfusion as measured by urine output, other organ perfusion, and metabolic acid-base status, using CVP monitoring to direct therapy. If organ perfusion cannot be maintained in the setting of adequate intravascular volume, administration of vasopressors and/or inotropes should be used to restore end-organ perfusion.93 Available evidence does not support the use of one particular vasopressor or combination of vasopressors. Once shock has resolved, patients should be managed with a conservative fluid strategy, with the goal of driving the CVP below 4 to keep each patient’s fluid balance net zero over their ICU stay.
Nutrition
Standard supportive care for the patient with ALI/ARDS includes providing adequate nutrition. The NIH NHLBI ARDS Network is currently conducting a randomized trial of trophic (10 mL/h, well below caloric requirements) versus full-calorie enteral feeds in patients with ALI/ARDS. The enteral route is preferred to the parenteral route and is associated with fewer infectious complications.111 Enteral feeding may also have other beneficial effects. Experimentally, lack of enteral feeding promoted translocation of bacteria from the intestine.112 In normal volunteers, administration of parenteral nutrition with bowel rest increased circulating levels of tumor necrosis factor alpha (TNF-α), glucagon, and epinephrine, and increased febrile responses compared to volunteers who received enteral nutrition.113
Until the results of the ARDS Network study become available, the goals of nutritional support in any critically ill patient include providing adequate nutrients for the patient’s level of metabolism and treating and preventing any deficiencies in micro- or macronutrients.114 Whether a particular dietary composition is beneficial in patients with ALI/ARDS is unclear. Immunomodulation via dietary manipulation has been attempted in critically ill patients, using various combinations of omega-3 fatty acids, ribonucleotides, arginine, and glutamine. A meta-analysis of these trials suggested a beneficial effect on infection rate but not on overall mortality.115 The ARDS Network recently conducted a large, multicenter, randomized placebo-controlled study of omega-3 fatty acid and antioxidant supplementation in patients with ALI/ARDS. This study was stopped early by the data safety monitoring board for a trend towards excess mortality in patients receiving the omega-3 fatty acid supplement (personal communication from Dr. Art Wheeler and Dr. Todd Rice). One other randomized controlled trial in ALI/ARDS studied the effects of an immunomodulatory nutritional formula.116 In that trial, a diet rich in fish oil, γ-linoleic acid, and antioxidants was associated with a shorter duration of mechanical ventilation and fewer organ failures, but no difference in mortality. Using a different approach, a high-fat, low-carbohydrate diet reduced the duration of mechanical ventilation in patients with acute respiratory failure.117 Although the mechanism of this beneficial effect was postulated to be due to reduction of the respiratory quotient and a resultant fall in carbon dioxide production, the most common cause of a high respiratory quotient in critically ill patients is not dietary composition but simply overfeeding.114 Overall, there is still no compelling evidence to support the use of anything other than standard enteral nutritional support, with avoidance of overfeeding, in patients with ALI/ARDS. There is evidence from one large study to suggest that omega-3 fatty acid and antioxidant supplementation may be deleterious, so this regimen is not recommended at present. How early to attempt institution of feeding remains an unanswered question.

Full access? Get Clinical Tree