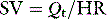Chapter 20 Acute heart failure
The pattern of heart failure seen in the community, outpatients clinics and specialist cardiac wards is dominated by the acute coronary syndromes and chronic heart failure, predominantly caused by ischaemic heart disease and hypertension.1,2 Heart failure is the commonest cause of hospital admission in people over 65 years of age and it has been estimated that in North America and Europe over 15 million patients have heart failure and 1.5 million new cases are diagnosed each year.3 Patients present with chest pain, shortness of breath, fatigue and oedema and will usually have single-organ failure. Management focuses on reducing cardiac work to relieve symptoms and prevent further myocardial damage.4,5
Patients either admitted with or who develop acute heart failure on the intensive care unit (ICU) frequently have overt or occult underlying coronary artery disease, but will usually have significant other organ dysfunction. Management in this setting focuses on both improving global and regional oxygen delivery and maintaining perfusion pressure, often with the use of drugs that stimulate rather than rest the myocardium.6–8 The resolution of this apparent paradox requires that for each patient management should attempt to achieve the frequently difficult balance between the best interests of the myocardium and the circulatory requirements of the other vital organs. The critical care physician should target the minimum necessary oxygen delivery and arterial pressure to maintain other organ function at maximum cardiac efficiency (e.g. ensuring adequate fluid resuscitation before starting beta-agonists) so that cardiac work and the risk of myocardial ischaemia and necrosis from exuberant beta-agonist use are minimised and the cardiologist should consider the wider circulation and other organ requirements when instituting strategies to protect the myocardium.
DIAGNOSIS OF ACUTE HEART FAILURE
The diagnosis of acute heart failure in critically ill patients can be more difficult than is commonly recognised. Although the underlying pathology in most patients with acute heart failure on intensive care will be coronary artery disease, other diagnoses must be considered (Table 20.1).
Table 20.1 Causes of acute heart failure on the intensive care unit
| Coronary artery disease |
| Infection – systemic sepsis,9 myocarditis |
| Mechanical – endocarditis, pulmonary emboli, valve problems, septal defects, tamponade, high intrathoracic pressure with inadequate preload |
| Drugs – beta-blockers, calcium antagonists, cytotoxic therapy |
| Hypoxaemia |
| Metabolic – acidaemia, thiamine deficiency, hypocalcaemia, hypophosphataemia |
| Myocardial contusion – blunt thoracic trauma |
| Myocardial infiltration – tumour, sarcoidosis, amyloidosis |
| Vasculitis – rare |
It is also important to reassess critically the patient referred with a diagnosis of acute heart failure to decide whether this is indeed the primary problem. The history, examination and initial investigations with routine blood tests, electrocardiogram (ECG) and chest X-ray may be compatible with this diagnosis but many such patients are elderly with multiple comorbidities and deciding whether the patient is suffering from a primary myocardial pathology as opposed to a pulmonary problem or indeed systemic sepsis9 can be difficult. Equally, patients believed to have a primary respiratory problem may fail to wean from ventilatory support because of a failure to realise that they have left ventricular failure with a high left atrial pressure and incipient pulmonary oedema, causing a reduction in pulmonary compliance, an increased work of breathing and respiratory distress when ventilatory support is withdrawn.
ECHOCARDIOGRAPHY (SEE CHAPTER 23)
Echocardiography is an extremely valuable investigation in the management of the critically ill patient with acute heart failure10 and the modern critical care physician should at least be able to perform a basic examination. It will frequently establish the underlying cardiac pathology and can be used to monitor the response to treatment. It will:
The images obtained with transthoracic echocardiography (TTE) may be poor in ventilated patients but the experienced operator can achieve considerable improvement using microbubble contrast techniques.11
MEASUREMENT OF TROPONIN AND BRAIN NATRIURETIC PEPTIDE
Myocardial injury and the development of acute heart failure are common but frequently unrecognised complications of critical illness occurring not only in patients with an overt acute coronary syndrome but also in other conditions such as sepsis and major pulmonary embolism (PE).12 Relying on blood tests alone to establish a diagnosis or to plan management is inadvisable but, when interpreted in conjunction with the wider clinical picture, brain-type natriuretic peptide (BNP) and cardiac troponin are two tests that are becoming routinely available and appear to be sensitive markers of myocardial stress and necrosis and to have significant prognostic significance.
BRAIN-TYPE NATRIURETIC PEPTIDE
BNP was first isolated from porcine brain but the major source is the ventricular myocardium. The main stimulus for synthesis and release is myocardial wall stress. As a triage tool in the emergency department it is able to discriminate patients with heart failure from those with pulmonary or other non-cardiac causes for acute dyspnoea and has been shown to reduce rates of ICU admission, length of hospital stay and cost.13 Several studies have demonstrated that, below a cut-off of 100 pg/ml, BNP has a sensitivity of almost 90% and a specificity approaching 80% as a test for excluding heart failure.14 It is now included in both the European and UK National Institute for Clinical Excellence guidelines for the management of heart failure15,16 and BNP has also been shown to be a marker of myocardial dysfunction and prognosis in severe sepsis.17
CARDIAC TROPONIN I AND T (cTnI, cTnT)
Troponin is part of the thin filament of the myocyte contractile apparatus and has three subunits: (1) I, which binds actin to inhibit actin-myosin contraction; (2) T, which binds tropomyosin to facilitate contraction; and (3) C, which binds calcium ions. The cardiac isoforms cTnI and cTnT are specific to the heart and can be measured in the blood after myocyte necrosis with 50% release by 4 hours, peaking at 12–24 hours and remaining elevated for up to 10 days. It is far more sensitive than the traditional cardiac enzyme tests such as creatine kinase and indeed it has substantially changed the diagnosis and management of acute myocardial infarction, as reflected in the new guidelines issued by the European Society of Cardiology15 and American College of Cardiologists. Troponin may be released in conditions other than acute coronary ischaemia18 such as sepsis and after chemotherapy and in the absence of evidence of myocardial necrosis as in acute heart failure or major PE, where it is believed that the acute ventricular dilatation causes increased membrane permeability. Raised troponin levels are also associated with increased morbidity and mortality in surgical ICU patients.19
In combination with BNP, cTnI is valuable in screening for massive PE: in massive and submassive PE both are raised and further investigation with computed tomography pulmonary angiogram (CTPA) is indicated but in minor PE both will be negative.
The remainder of this chapter addresses the assessment and principles of management of ventricular function in patients admitted to the ICU with acute heart failure. This inevitably involves reference to circulatory failure and the state of the peripheral circulation but the more detailed aspects of oxygen delivery and control of the regional and microcirculation are considered elsewhere20 (see Chapters 11 and 12), as are the acute coronary syndromes2 (see Chapter 16) and chronic heart failure.1
CIRCULATORY FAILURE OR ‘SHOCK’
Failure to maintain an adequate oxygen supply to the tissues with the consequent development of anaerobic cellular metabolism defines circulatory failure or ‘shock’, a term that benefits from brevity but little else since it implies neither cause nor prognosis, but its use is now widespread and inescapable. Table 20.2 classifies circulatory ‘shock’.
Table 20.2 Major categories of circulatory failure or ‘shock’
| Cardiogenic | Myocardial infarction, myocarditis, vasculitis, valve dysfunction (e.g. critical aortic stenosis, mitral regurgitation, acute endocarditis), post cardiac bypass surgery, drug overdose (β-blockers, calcium antagonists) |
| Hypovolaemic | Haemorrhage, burns, gastrointestinal fluid loss |
| Obstructive | Pulmonary embolus, cardiac tamponade, tension pneumothorax |
| Anaphylactic | Drugs, blood transfusion, insect sting |
| Septic | Bacterial infection, non-infective inflammatory conditions, e.g. pancreatitis, burns, trauma |
| Neurogenic | Intracranial haemorrhage, brainstem compression, spinal cord injury |
In considering these causes of circulatory failure, several points require emphasis:
ASSESSMENT OF VENTRICULAR FUNCTION
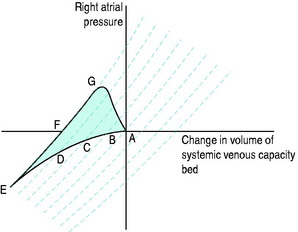
Table 20.3 illustrates typical values in normal subjects and in the common causes of circulatory failure with calculation of the associated vascular resistances and oxygen delivery. The values quoted are merely examples that indicate the pattern of circulatory derangement produced by these pathologies: pre-existing cardiopulmonary disease and the severity of the condition will affect the precise figures obtained in individual cases and the response to vasoactive therapy.
Table 20.3 Measurements in a normal 75-kg adult and in various conditions causing circulatory ‘shock’
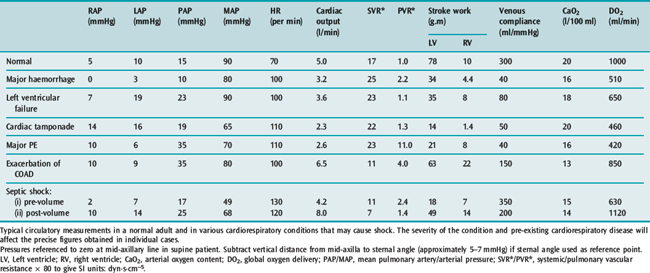
Stroke volume (SV) is calculated from CO and heart rate:
Three factors determine stroke volume: (1) preload; (2) afterload; and (3) myocardial contractility.
VENTRICULAR PRELOAD
Ventricular preload, traditionally assessed from the atrial filling pressures, determines the end-diastolic ventricular volume, which, according to Starling’s law of the heart and depending on ventricular contractility, dictates the stroke work generated by each ventricle at the next cardiac contraction. The resulting stroke volume depends on the resistance or afterload that confronts the ventricle.26
On the general ward the jugular venous pressure (JVP) is measured from the sternal angle but in ICU vascular pressures are measured from the mid-axillary line in the fifth intercostal space. From this reference point, in the supine position, the normal RAP is between 4 and 8 mmHg and the LAP, or wedge pressure, is between 8 and 12 mmHg. Relative changes in either the contractility of the two ventricles or the respective vascular resistances will change the relationship between the atrial pressures, which must then be independently assessed.27
The systemic venous bed is the major intravascular capacitance or reservoir of the circulation with a compliance that can vary from 30 to over 300 ml/mmHg and which provides a buffer against the effects of intravascular volume loss. It also explains the response observed in major haemorrhage and subsequent transfusion. As volume is lost, venous tone increases, preventing the large falls in atrial filling pressures and CO that would otherwise occur. If the equivalent volume is returned over the subsequent few hours the RAP gradually returns to normal as the intravascular volume is restored and the reflex increase in sympathetic tone abates. However, rapid reinfusion of the same volume does not allow sufficient time for the venous and arteriolar tone to fall and may result in the LAP rising to a level that precipitates pulmonary oedema, although the intravascular volume has only been returned to the prehaemorrhage level and left ventricular function is normal (Figure 20.1).

Full access? Get Clinical Tree