with increased anion gap (e.g., increase in strong nonvolatile acids). A normal anion gap is 7 to 14 mEq/L.
![]()
Causes: Renal failure, ketoacidosis, lactic acidosis, nonketotic hyperosmolar coma, alcohol, inborn errors of metabolism, salicylates, methanol, ethylene glycol, paraldehyde, toluene, sulfur, rhabdomyolysis
Treatment: Treat the underlying process and correct respiratory acidosis by initiating hyperventilation with target PaCO2 in the low 30s. If pH remains below 7.20, sodium bicarbonate (NaHCO3) can be given intravenously (IV) at a dose of 1 mEq/kg. Notably, the HCO3− in sodium bicarbonate will cause a transient increase in PaCO2, underscoring the importance of controlled ventilation. If acidosis remains uncorrected, the patient must be dialyzed. A pH above 7.25 generally negates the physiologic effects of acidemia.
Targeted Therapies
• Diabetic ketoacidosis: Fluid resuscitation to replace deficit from hyperglycemic osmotic dieresis, insulin drip, and replacement or monitoring of electrolytes (potassium, magnesium, and phosphate)
• Lactic acidosis: Restore tissue perfusion and oxygenation
• Salicylate overdose: Alkalinization of urine (pH >7.0) with NaHCO3 to facilitate excretion
• Ethanol and ethylene glycol toxicity: Ethanol infusion or fomepizole administration, which acts by competitively inhibiting alcohol dehydrogenase; consider hemodialysis or hemofiltration.
Anesthetic Implications of Metabolic Acidosis:
• Potentiation of sedative effects of opioids
• Depressed airway reflexes
• Cardiovascular depression
• Increased arrhythmogenicity of halothane
• Elevated potassium levels from acidosis may preclude the use of succinylcholine.
Case Card
A patient presents to the emergency department after ingesting ethylene glycol. Which of the following acid–base disturbances would you expect see?
A. Anion gap metabolic acidosis
B. Non–anion gap metabolic acidosis
C. Respiratory acidosis
D. Metabolic alkalosis
E. Respiratory alkalosis
Case Card Answer
A. Anion gap metabolic acidosis
Ingestion of ethylene glycol results in an anion gap metabolic acidosis. The ingestion of ethylene glycol (which is an acid) results in an increase in the unmeasured cations in the following equation:
![]()
Diagnostic Approach
Arterial samples are the most commonly analyzed, but venous blood can be used with some limitations.
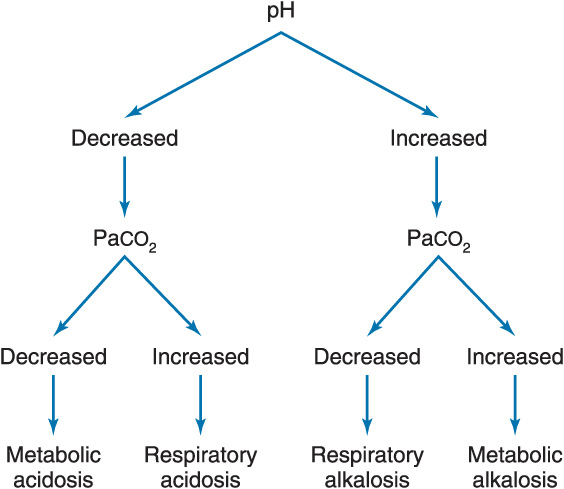
Mixed Acid–Base Disorders (present if the change in pH is not as follows)
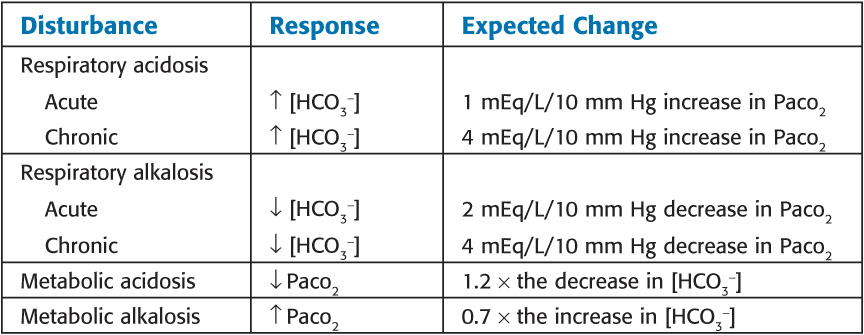
In a respiratory disorder, the pH changes 0.08 units in the opposite direction for every 10 mm Hg change in PaCO2.
In a metabolic disorder, every 6 mEq change in HCO3 changes the pH 0.1 unit in the same direction.
Normal Compensatory Responses
Metabolic Alkalosis
Definition: Primary increase in plasma ![]()
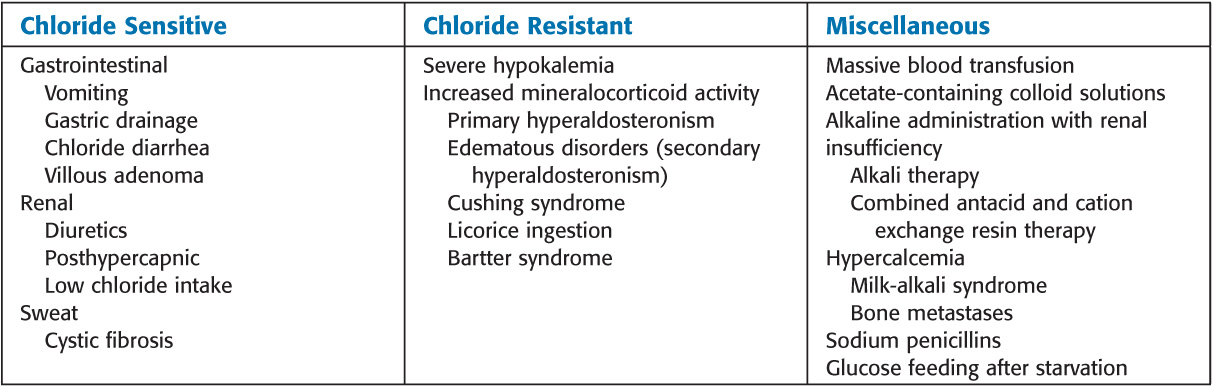
Causes
Treatment: Treat the underlying disorder.
• Chloride sensitive: IV normal saline and potassium chloride
• Excessive gastric fluid losses: H2 blockers
• Concurrent edema: Acetazolamide
• Increased mineralocorticoids: Aldosterone antagonists such as spironolactone
• If pH is above 7.60, IV hydrochloric acid, ammonium chloride, arginine hydrochloride, or hemodialysis may be considered.
Anesthetic implications:
• Cardiac dysrhythmias when combined with hypokalemia
• Potentiation of neuromuscular blockade
• Left shift of oxygen dissociation curve (increased affinity of O2 for hemoglobin)
• Hypokalemia (hydrogen ions shift extracellularly in exchange for potassium ion)
• Decreased calcium ion concentration, which leads to circulatory depression and neuromuscular irritability
• Cerebral ischemia from decreased cerebral blood flow during respiratory alkalosis, especially during hypotension.

Full access? Get Clinical Tree





