Key Clinical Questions
What are common causes of increased anion gap metabolic acidoses?
How is the urinary anion gap used in the diagnosis of normal anion gap metabolic acidoses?
What are the major types of renal tubular acidosis, and how are they diagnosed?
How is urinary chloride used in the diagnosis of metabolic alkalosis?
How is the ΔGap used to diagnose complex acid-base disorders?
Introduction
Acid-base disturbances occur frequently in the acutely ill hospitalized patient. Many physicians are intimidated by the complexity of acid-base analysis, as multiple, partially offsetting disorders can be challenging to diagnose. However, in the hospital setting, especially the critically ill, it is crucial that patients with acid-base disturbances be quickly identified and the abnormality be accurately interpreted. Swift intervention to treat the underlying causes is often necessary to avoid the often lethal consequences of severe acid-base disturbances.
Normal Acid-base Homeostasis
A typical Western diet generates about 15,000 mmol of volatile acids, in the form of CO2, and ˜ 70 mmol (1 mmol/kg) of fixed acid each day. CO2 is excreted by normal respiration. Fixed acids are buffered by intra- and extracellular buffers. New buffers, predominantly HCO3−, must be continuously produced to replace buffers consumed in titrating fixed acids. Complex acid-base homeostasis mechanisms, which include chemical buffering in conjunction with the excretion of CO2 by the respiratory system and new HCO3− production by the kidneys, normally maintain the blood pH between 7.35 to 7.45.
The kidney plays a central role by reabsorbing all of the filtered bicarbonate, approximately 4000 mmol per day, and also generating new bicarbonate. The central nervous system and the respiratory systems control the arterial CO2 tension (PaCO2). Under normal steady-state conditions, the H+ balance is preserved by intricate mechanisms which ensure that the net quantity of acid secreted and the consequent renal generation of new bicarbonate equals the rate of metabolic proton generation. A disturbance of this fine balance results in an acid-base disorder.
Glossary of terms for acid-base disorders
|
Simple Acid-base Disorders
These disorders involve only a single acid-base disorder, and include metabolic acidosis, metabolic alkalosis, respiratory acidosis, and respiratory alkalosis. A key distinguishing feature is that the pH is always abnormal, as the compensation is never complete. Simple acid-base disorders can be classified as acute or chronic, based on the degree of compensation (Table 245-1).
| Abnormality | Compensation |
|---|---|
| Metabolic acidosis | PaCO2= (1.5 × HCO3−) + 8 ± 2 |
| Metabolic alkalosis | For each 10 mEq/L increase in HCO3−, PaCO2 increases by 6 mm Hg |
| Respiratory acidosis | |
| Acute | For each 10 mm Hg increase in PaCO2, HCO3− increases by ˜ 1 mEq/L |
| Chronic | For each 10 mm Hg increase in PaCO2, HCO3− increases by ˜ 4 mEq/L |
| Respiratory alkalosis | |
| Acute | For each 10 mm Hg increase in PaCO2, HCO3− decreases by ˜ 2 mEq/L |
| Chronic | For each 10 mm Hg increase in PaCO2, HCO3− decreases by ˜ 4 mEq/L |
|
Mixed Acid-base Disorders
When multiple acid-base disorders coexist independently and are not merely as compensatory responses, they are classified as mixed acid-base disorders. These are not uncommon in the critically ill patient, and can lead to dangerous extremes of pH and a poor outcome. However, the pH may also be normal or near normal when acidosis and alkalosis coexist in the same patient. A patient with diabetic ketoacidosis who also has metabolic acidosis resulting from chronic kidney disease is an example of a mixed acid-base disorder. Triple acid-base disorders are not uncommon, and are often not recognized unless until the clinician specifically considers this possibility. For example, a patient with metabolic acidosis due to lactic acidosis complicating severe sepsis may develop metabolic alkalosis from intravenous bicarbonate administration, with superimposed respiratory alkalosis due to hyperventilation from mechanical ventilation. Another example is the patient with respiratory acidosis due to altered consciousness, metabolic acidosis due to diabetic ketoacidosis, and metabolic alkalosis from vomiting may have an identical arterial blood gas. It is critical to always interpret acid-base abnormalities in the clinical context of the patient.
| Common acid-base examples in the hospital setting | |
|---|---|
| Respiratory alkalosis | Anxiety, pain, hypoxia, sepsis, acute or chronic liver disease, mechanical ventilation, pregnancy, drugs (salicylates, progesterone, catecholamines), central nervous system disease |
| Acute respiratory acidosis | Acute airway obstruction, narcotic analgesics, severe pneumonia, severe pulmonary edema, hemothorax, pneumothorax, flail chest, ventilator dysfunction |
| Neuromuscular disorders and central nervous system depressants | |
| Chronic respiratory acidosis | Chronic lung disease, central hypoventilation |
| Chronic neuromuscular disorders | |
| Metabolic alkalosis | Low urinary chloride: vomiting, nasogastric suction, past diuretic use, posthypercapnia |
| Normal or high urinary chloride: primary or secondary hyperaldosteronism, diuretic use, excess alkali, refeeding | |
| Note: a PCO2 > 55 mm Hg suggests additional primary respiratory abnormality. | |
| Metabolic acidosis | Elevated anion gap: ketoacidosis, renal failure, lactic acidosis, rhabdomyolysis, toxins |
| Excess anion gap (total anion gap − normal anion gap of 12) + measured bicarbonate concentration: If > normal serum bicarbonate range, then coexisting underlying metabolic alkalosis | |
| If < normal serum bicarbonate range, then coexisting underlying nongap metabolic acidosis | |
| Normal anion gap: posthypocapnia, GI or renal losses, hyperchloremic acidosis (as in the recovery phase of DKA, due to failure to regenerate bicarbonate from ketoacids lost in urine) | |
| Respiratory acidosis, metabolic acidosis, and metabolic alkalosis: low pH, high PCO2, low HCO3 | Obtunded patient (respiratory acidosis) with lactic acidosis (metabolic acidosis) and vomiting (metabolic alkalosis) |
Approach to Acid-base Disorders
The first step in interpreting acid-base disorders is to perform a detailed history and physical examination. Next, one should simultaneously measure arterial blood gas (ABG) and plasma chemistries. Blood gas analyzers directly measure the pH and PaCO2; the bicarbonate value reported in an ABG analysis is calculated based from the pH and PaCO2. Blood bicarbonate concentration is measured in the metabolic panel as total dissolved CO2, which is ˜95% bicarbonate. The samples can be validated by comparing the calculated HCO3− value reported on the arterial blood gas measurement with the measured HCO3− value on the chemistry panel. If the difference between the two values is greater than 2 mmol/L, the samples may not have been drawn simultaneously, or a laboratory error may be present. Excessive heparin in the syringe used to obtain the arterial blood sample can also cause confounding results.
Stepwise approach to acid-base disorders
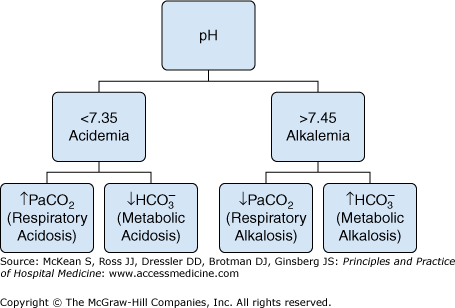
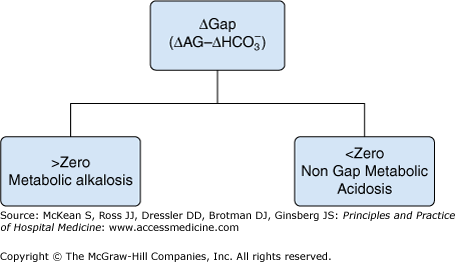
|
Next, identify the primary acid-base disorder by looking at the arterial pH, HCO3− and PaCO2 (Table 245-2). If either respiratory acidosis or alkalosis, then determine whether the condition is acute or chronic from the change in the serum bicarbonate (Table 245-1). Finally calculate the anion gap (AG) and ΔAG (see below). Further evaluation will be guided by the type of acid-base disorder.
Important formulae for solving acid-base problems
|







