Patricia Jordano An abdominal aortic aneurysm (AAA) is a progressive, permanent, localized dilation of the abdominal aorta with aortic diameter of 3.0 cm or more, or a 50% increase in diameter compared with the adjacent normal segment.1–3 The aorta is a conduit that carries blood to the body. The aorta is divided by the diaphragm into the thoracic and abdominal aorta. It is composed of three layers: the tunica intima, tunica media, and tunica adventitia. In adults, the normal diameter of the abdominal aorta varies with age, height, gender, and body habitus, but the average infrarenal aortic diameter in an adult is approximately 2.0 cm and typically less than 3.0 cm. The prevalence of an AAA located in the infrarenal section of the aorta is at least three times greater than a thoracic aortic aneurysm.4 Aneurysms are described by their shape, which help identify a true aneurysm. A true aneurysm involves all three layers of the aorta. The more common, fusiform aneurysm is a symmetric weakness of the entire circumference of the aorta that produces a bulge. A saccular aneurysm is an asymmetric weakness or bleb on the side of the aorta; these defects result from trauma or an internal wall defect caused by an ulcer. A pseudoaneurysm, or false aneurysm, is an enlargement of only the outer layer of the blood vessel wall. AAAs are also described by size (a small aneurysm has a diameter <4.0 cm, a medium aneurysm has a diameter of 4.0 to 5.4 cm, a large aneurysm has a diameter ≥5.5 cm, and a very large aneurysm has a diameter ≥6.0 cm), as well as involvement of the renal or visceral vessels. AAA is an important clinical diagnosis because it is associated with considerable risk of rupture and death as the aneurysm enlarges to a diameter of more than 5.0 cm (1.96 inches).2,5 In the United States, 15,000 deaths per year are attributed to AAAs, and it is the 10th leading cause of death in men older than 55 years.6–8 Treatment is usually recommended when an AAA grows to larger than 5.5 cm in diameter. Most patients who have a ruptured AAA will die before reaching the hospital, and of those who make it to the hospital and have surgery, the outcome is dependent on their presenting clinical condition, but typically the mortality rate has been stated as anywhere from 50% to 80%. The high mortality rate has not changed over the past 20 years despite improvement in operative technique and preoperative management.2,5,8 Aortic aneurysms are a complex disease with genetic and environmental risk factors. Major risk factors for AAA include advancing age (>65), male gender, family history of an AAA, and cigarette smoking (current or past). Additional risk factors include atherosclerotic vascular disease, hypertension, hyperlipidemia, and other vascular aneurysms (e.g., iliac, femoral, popliteal aneurysms).2,3,5,9–11 The association between chronic obstructive pulmonary disease (COPD) and AAA remains elusive. The high prevalence of AAA in patients with COPD may be related to medications (oral steroids) and coexisting disease rather than to a common pathway of pathogenesis involving plasma elastase or α1-antitrypsin deficiency.10–12 Those treated with corticosteroids have a more rapid rate of AAA expansion. Individuals younger than age 60 are not as affected by AAA. Males are affected more than females at a 6:1 ratio.2,5,9–11 Smoking is the greatest environmental risk factor for AAA development. An AAA is over seven times more likely to develop in a smoker than a nonsmoker, with duration of smoking being a key variable.5 First degree male relatives of patients with AAA have two to four times the normal risk for AAA. Female first-degree relatives appear to have similar risk, but the data are less certain.2,11 Those with a decreased risk of AAA development include women, non-Caucasians, and diabetics.1,2,5,9,11 Factors associated with an increased risk of rupture include female gender, large initial aneurysm diameter, low forced expiratory volume in 1 second (FEV1), current smoking, and elevated blood pressure.2,5,11 Risk factors for aneurysm development, expansion, and rupture are listed in Table 117-1. AAA is a disease of the medial wall layer of the aorta. It is characterized by degeneration of the extracellular matrix proteins and the presence of an inflammatory cell infiltrate composed predominantly of T cells. Degradation of the cell wall proteins in the medial layer occurs as a result of complex interactions among genetic factors, inflammatory cytokines, matrix metalloproteinases (MMPs), tissue inhibitors of MMPs, and others. The consequences include dissolution and fragmentation of collagen and elastin, leading to expansion of the vessel wall.2–5,9 When the aortic wall tension exceeds the tensile strength of the wall collagen and the wall can no longer withstand the repetitive force of systolic contraction, the aneurysm ruptures. Although an AAA may cause symptoms as a result of the pressure on surrounding structures, about 75% are asymptomatic at initial diagnosis.13,14 Asymptomatic AAAs are generally detected during an incidental radiologic or surgical procedure. Alternatively, in thin patients, a supine abdominal examination may readily show a pulsatile abdominal mass. Thromboembolic phenomena may herald the presence of an AAA. Microembolic infarcts in the lower extremity of a patient may suggest either abdominal or popliteal aneurysm. Embolization of mural thrombus from an abdominal aneurysm may be seen with acute limb ischemia caused by femoral or popliteal occlusion.13 The classic diagnostic triad of ruptured AAA is hypotension, pulsatile abdominal mass, and abdominal pain or back pain. The triad is encountered in less than 50% of patients with a ruptured AAA.14 Symptoms of AAA may include a sensation of abdominal discomfort, back pain, pulsation of abdomen, or flank pain.5,14 Less frequently, individuals may complain of pain in the legs, chest, or groin area. They may also report anorexia, nausea, vomiting, or dyspnea. In a patient with a history of aneurysm or pulsatile mass, abdominal pain must be considered to represent a rapidly expanding or ruptured aneurysm and must be treated accordingly. Palpation of the abdomen for AAA is one of the few physical examination maneuvers that is an evidence-based recommendation in the periodic health examination of older men.15 For detection of AAA on physical examination, the patient is positioned supine with knees flexed to relax the abdominal wall. The examiner places the palm over the epigastrium to detect a transmitted pulsation. The examiner then places both hands on the abdomen with palms down and an index finger on either side of the pulsating area to measure the aortic width. An aneurysm expands laterally with each systole. An AAA is suspected when the aorta is judged to be at least 3.0 cm ( Unfortunately, only 30% to 40% of aneurysms are noted on physical examination, with detection dependent on the skill of the examiner and the size of the aneurysm. The sensitivity of abdominal palpation increases with AAA diameter, from 29% for AAAs of 3.0 to 3.9 cm to 76% for AAAs of more than 5.0 cm. The sensitivity of abdominal palpation also increases (91%) when the abdominal girth is less than 100 cm (40-inch waistline) compared with 53% when abdominal girth is 100 cm or greater. Overall, when the girth is less than 100 cm and the AAA is more than 5.0 cm, abdominal palpation is highly sensitive (100%) for detection of AAA.14,15 If on examination one finds a pulsatile mass in the groin or popliteal fossa, this raises suspicion for an AAA, because multiple aneurysms often coexist.5,14 Given the high mortality associated with emergent AAA repair, early detection and repair before rupture are the mainstay of AAA management. The American College of Cardiology (ACC) and American Heart Association (AHA) practice guidelines for management of peripheral arterial disease recommend that “Men 60 years of age or older who are either a sibling or offspring of patients with AAAs should undergo physical examination and ultrasound screening for detection aortic aneurysms.” And “men who are 65-75 years of age who have ever smoked should undergo a physical examination and a 1-time ultrasound screening for detection of AAAs.”11 The U.S. Preventive Services Task Force (USPSTF) recommendation statement recommends “1-time screening for AAA with ultrasonography in men aged 65-75 years who have ever smoked.”16 The USPSTF recommends that clinicians selectively offer screening for AAA in men aged 65 to 75 who have never smoked rather than routinely screening all men in this group. The Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act was approved by the United States Congress in January 2007. The SAAAVE Act permits a single screening aortic ultrasound examination as part of the “Welcome to Medicare” package for patients with defined risk factors for AAA. Males aged 65 to 75 years who have smoked more than 100 cigarettes in their lifetime or patients of any age or either sex with a strong family history are eligible for this screening examination.2,17,18 Ultrasonography is the imaging study ordered most often for screening and initial confirmation of an aneurysm. It can measure anteroposterior, transverse, and longitudinal dimensions of an AAA. Ultrasonography can provide a reasonably accurate measurement of initial size and can be used for serial follow-up evaluation.5,14,19 This modality is widely available, is painless, does not expose the patient to ionizing radiation, and is inexpensive. It can also visualize important anatomic markers such as relation of major arterial branches and adjacent organs. Duplex ultrasound can provide additional information on aortic flow. Computed tomography angiography (CTA), often with three-dimensional imaging, is the preferred and most widely used imaging modality before aortic aneurysm repair. It accurately demonstrates dilation of the aorta and the relationship to major branch vessels, both proximally and distally. It will show the degree of calcification, presence of mural thrombus, inflammatory aneurysms, aneurysmal leakage, penetrating aortic ulcer, length of the aneurysm neck, iliac artery, and whether other organs have become displaced.14,19 It is noninvasive but does expose the patient to ionizing radiation and contrast medium, which can be harmful, especially in patients with kidney disease. Standard contrast aortography is infrequently used but may be indicated in select individuals, including those with suspected suprarenal extension, suspected visceral or renal artery disease, iliofemoral occlusive disease, horseshoe kidney, prior aortic or colonic surgery, and unusual aneurysms (e.g., mycotic, aortocaval fistula).19 The procedure uses ionizing radiation and contrast. Most institutions, however, are using CTA or magnetic resonance imaging (MRI) or magnetic resonance angiography (MRA) for the preoperative evaluation of AAA. MRI and MRA may also be used to diagnose aortic disease and for preoperative planning. MRI and MRA have limitations, including inability to be used for patients with pacemakers or other metallic hardware that would affect the magnetic field. In addition, for certain patients, claustrophobia or unstable medical conditions would preclude their being in the tube during the necessary acquisition time. The advantage of MRI or MRA is its absence of iodinated contrast material and radiation exposure. The use of gadolinium is contraindicated in patients with renal failure. With pre–endovascular aneurysm repair (EVAR) planning, contrast-enhanced MRA is comparable to CTA. The differential diagnoses for AAA include conditions associated with abdominal pain or back pain. These conditions are listed in the differential diagnosis box. CT is the most readily available method to rule out alternative causes of abdominal pain.
Abdominal Aortic Aneurysm
Definition and Epidemiology
Pathophysiology
Clinical Presentation
Physical Examination
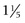 inches) in maximum diameter. Auscultation may reveal a bruit over the mass, but abdominal bruits are not specific for AAA formation.
inches) in maximum diameter. Auscultation may reveal a bruit over the mass, but abdominal bruits are not specific for AAA formation.
Diagnostics
Differential Diagnosis
Abdominal Aortic Aneurysm
Chapter 117








