CHAPTER 32. Care and Transport of the Neonate
Christine Tijerina
Competencies
Get Clinical Tree app for offline access
1. Perform an initial assessment of the neonatal patient.
2. Perform the necessary interventions to maintain the neonate’s airway, breathing, and circulation after delivery.
3. Recognize the normal transitional stages to extrauterine life and identify a neonate’s inability to transition appropriately.
4. Prepare the neonate for transport with use of appropriately sized equipment.
5. Maintain the same or higher level of care during transport.
The neonatal patient is defined as any newborn infant less than 28 chronologic days of age or 28 days beyond the due date in the case of preterm infants. A term pregnancy is typically 38 to 42 weeks. Premature infants are commonly defined as infants born before 37 weeks gestation, and postterm infants are born later than 42 weeks gestation.
Neonates have unique anatomy, physiology, and pathophysiology that require advanced knowledge and understanding of these differences for appropriate care. The depth of knowledge of neonatal specialty needed by transport personnel is directly related to the mission profile of the team. Medical transport providers who care for neonates in any out-of-hospital environment must have training in the stabilization and care of the types of infants they may transport. Attention to both appropriate team composition and the availability of specialized equipment and medications is necessary to ensure safe transport of these patients.
Medical transport providers may care for neonates in a prehospital setting or an interfacility transport setting. For neonatal patients born or presenting in a nonmedical environment, such as a home or car, the immediate stabilization needs of the infant (thermoregulation, resuscitation, stabilization, and expedient transport to the nearest appropriate medical facility) are stressed. Interfacility transport of neonates requires an emphasis on maintaining the equivalent level of care or a higher level of care during stabilization and transport to the receiving hospital. Competency in neonatal care protocols and procedures, and recognized expertise in the field, is an essential component of neonatal transport care.
The American Academy of Pediatrics offers some specific criteria for the composition and training of the neonatal transport team. In addition, this organization also provides guidelines for the minimum equipment that should be used for safe stabilization and transport of the neonate. Box 32-1 includes a summary of Neonatal Transport Guidelines.
BOX 32-1



Guidelines for Neonatal Transport from the American Academy of Pediatrics
1. Access to neonatal-pediatric transport services is essential to the health of infants and children.
2. Requirements for neonatal-pediatric interfacility transport should include: patient and team safety, a level and quality of patient care equivalent to the care provided in a critical care unit, quality monitoring, optimal resource allocation, cost effectiveness, and rapid response.
3. Skilled health care professionals.
4. Adequate volume of patients so that the team remains competent.
5. Maintenance of a database that must include demographic data, treatment outcomes, and quality improvement data.
6. Communication between the transport system and the communities they serve must be open and expeditious at all times.
7. Encourage transport back to the referring facility when the infant’s clinical condition allows it.
Modified from: American Academy of Pediatrics: Guidelines for air and ground transport of neonatal and pediatric patients, Elk Grove Village, IL, 2006, AAP.
FETAL CIRCULATION AND TRANSITION
The scope of this chapter allows only a brief overview of fetal circulation and transition to extrauterine life. In utero, the placenta and fetus are nourished by an umbilical vein that carries highly oxygenated blood to the right atrium via the ductus venosus and the inferior vena cava. The right and left ventricles both pump at systemic pressures into the aorta (Figure 32-1). 15 A large percentage of this blood is directed across the foramen ovale to the left atrium, left ventricle, and ascending aorta to perfuse the coronary arteries and the brain with the most highly oxygenated blood in fetal circulation. Some of the blood from the umbilical vein along with blood returning from the superior vena cava flows through the tricuspid valve to the right ventricle and out through the pulmonary valve. Most of the blood flow from the right ventricle shunts from the pulmonary artery through the ductus arteriosus and into the descending aorta as a result of the high pressure of the pulmonary vasculature system. The shunted blood mixes with the remainder of the blood coming from the left side of the heart.
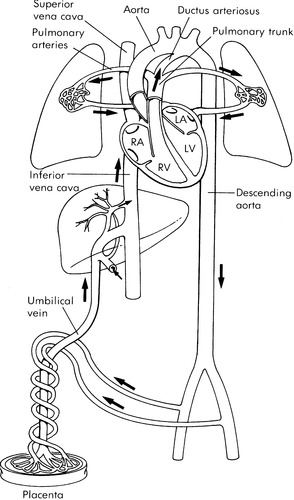 |
| FIGURE 32-1 Normal fetal circulation and major fetal flow patterns. (From Heymann MA: Biophysical evaluation of fetal status: fetal cardiovascular physiology. In Creasy RK, Resnik R, editors: Maternal fetal medicine, Philadelphia, 1984, Saunders.) |
The transition to extrauterine life begins the moment the neonate takes its first breath. The expansion of the lungs and exposure to oxygen at birth causes the pulmonary vascular resistance to fall and allows a rapid increase in pulmonary blood flow and a consequent decrease in flow across the ductus arteriosus. Simultaneously, as the umbilical cord is clamped, the low-resistance placental circuit is removed and an increase in systemic resistance occurs. This increase in afterload, and the increased return to the left atrium from the pulmonary circuit, helps to close the flap-like foramen ovale.
Careful ongoing assessment and early intervention are critical during this time period because subtle changes can indicate undiagnosed congenital cardiac diseases. Neonatal hypoxia, hypoglycemia, hypothermia, sepsis, stress, and acidosis can all interfere with the normal progression of this transition period. 9,23,24 Common findings at this time may include intermittent grunting, mild retracting, and tachypnea and poor feeding. 31 The infant must be observed and monitored closely until all these symptoms have been addressed or resolved.
INITIAL PRIORITIES: DELIVERY ROOM MANAGEMENT
Assessment of the Infant
The initial assessment should be brief and focus on identification and treatment of life-threatening issues related to airway, breathing, or circulation. One essential component of the initial assessment is the Apgar score. The Apgar score, developed in 1953 by Dr Virginia Apgar (Table 32-1), 1 is a basic rapid evaluation of the infant’s immediate adaptation to extrauterine life. Evaluation of color, respiratory effort, heart rate, body tone, and responsiveness to stimuli provides an immediate guidance of how the transition to extrauterine life is progressing. Apgar scores are measured at 1 and 5 minutes after birth. Early studies correlated low 5-minute Apgar scores with poor neurologic outcome8; however, later studies have shown that the Apgar score is not an accurate indicator of neonatal asphyxia as defined by metabolic acidosis. 22,32 If, however, the neonate’s condition continues to be depressed after 5 minutes of age, continuation of Apgar assignment for up to 20 minutes after delivery while necessary care interventions are provided can be useful. 20
| Sign | |||
|---|---|---|---|
| Score | 0 | 1 | 2 |
| Appearance, color | Blue, pale | Centrally pink | Completely pink |
| Pulse, heart rate | None | Less than 100 beats/min | Greater than 100 beats/min |
| Grimace, reflex | No response | Grimace | Cough, gag, cry |
| Activity/attitude | Flaccid/limp muscle tone | Some flexion | Well-flexed/active motion |
| Respiratory, effort | None, irritability | Weak/irregular | Good, crying |
Clearing the Airway
The airway should be cleared with either bulb syringe, DeLee, or a 10F to 14F suction catheter with use of a mechanical suction immediately on delivery of the neonates head and before the infant’s first breath. The oropharynx should normally be cleared before suctioning of the nares because stimulation may cause the infant to gasp and aspirate secretions present in the oropharynx. Stimulation of the vagus nerve from suctioning too vigorously and too deeply may result in severe bradycardia; therefore, suctioning after the initial clearing of the airway should be done on an as-needed basis. The Neonatal Resuscitation Program (NRP) recommends that no greater than 100 mm Hg of negative pressure should be used to avoid injury to the neonate. 20
Aspiration of meconium-stained fluid into distal airways may significantly contribute to morbidity and mortality. Infants presenting with depressed condition with inadequate respiration, poor tone, or a heart rate (HR) less than 100 beats/min should have the trachea intubated and suctioned with an endotracheal tube and a meconium aspirator before positive-pressure ventilation. If the baby looks good (i.e., breathing well, HR > 100 beats/min, good muscle tone) and is covered in meconium, suctioning of only the mouth and nose may be prudent. The NRP states “Some previous recommendations have suggested that endotracheal suctioning should be determined by whether the meconium has ‘thick’ versus ‘thin’ consistency. While it might be reasonable to speculate that thick meconium might be more hazardous than thin, there are currently no clinical studies that warrant basing suctioning techniques on meconium consistency.”20 The incidence of meconium aspiration syndrome can be greatly reduced with effective clearing of the airway of affected infants. 6,34
Initiation of Breathing
Most neonates initiate spontaneous breathing without intervention. Neonates that do not initiate breathing on their own may require only minimally invasive interventions such as opening the airway and positioning to stimulate breathing. Neonates that are breathing spontaneously but remain centrally cyanotic may need initial oxygen support delivered with blow-by oxygen, face mask, or nasal prongs. If the neonate does not begin spontaneous effective respiration or the heart rate remains below 100 beats/min after opening and clearing the airway, stimulating the infant and providing supplemental oxygen with assistive manual ventilations should be initiated at a rate of 40 to 60 breaths/min with the minimal amount of oxygen to support oxygenation. Adequate ventilation should be evaluated with auscultation of breath sounds and observation of chest excursion and heart rate. The infant should respond to adequate ventilation with an improvement in color, heart rate, and tone.
Neonates are at high risk for pulmonary air leaks, thus ventilating pressures should always be monitored with a manometer. Although pressures up to 30 to 40 cm H 2O may be initially necessary to open the lungs, the lowest pressures possible to maintain adequate chest rise and oxygenation should be used. Once the infant has established spontaneous respiration and a heart rate of 100 beats/min and is centrally pink, the team should reevaluate the amount of support needed.
If no response in heart rate and color is seen after 30 seconds of assistive manual bag and mask ventilation, then cardiopulmonary resuscitation (CPR) should be initiated while preparations for endotracheal tube (ETT) intubation are made. Because the placement of the endotracheal tube leaves little room for error, careful and immediate evaluation for right or left mainstem or esophageal intubation should be done and adequacy of ventilation should be assessed. In addition to the presence of improved color, increasing heart rate indicating successful intubation, confirmation of ETT placement with the use of a color metric CO 2 detector is recommended to confirm ETT placement. 20Table 32-2 contains a guideline of endotracheal tube size and insertion depth.
| Weight (kg) | Endotracheal Tube (size) | Depth of Insertion (cm from upper lip) | Suction Catheter (size) |
|---|---|---|---|
| 1 | 2.5 | 7 | 5F |
| 2 | 3.0 | 8 | 6F |
| 3 | 3.5 | 9 | 8F |
| 4 | 4.0 | 10 | 8F-10F |
Chest Compressions
Chest compressions should be initiated if the heart rate remains below 60 beats/min or does not increase after 30 seconds of assisted manual ventilation. Two methods are recommended for chest compressions on the neonate: the thumb technique and the two-finger technique. Resuscitation for neonates should be performed at a rate of 120 “events” per minute. Ninety compressions plus 30 breaths should occur. The status of the infant should be reassessed after 30 seconds of resuscitation. If the child’s heart rate is above 60 beats/min, compressions can be stopped. If chest compressions are not effective, epinephrine should be administered. 20
The thumb technique involves the caregiver encompassing the infant’s chest with both thumbs used to depress the infant’s sternum. The baby should be placed on a firm surface for delivery of effective compressions. The two-finger technique uses the tips of the caregiver’s middle finger and either the index finger or the ring finger of one hand to compress the sternum. The other hand can be used to support the infant’s back. With both the thumb and the two-finger technique, the infant’s chest should be depressed to a depth of approximately one third of the anterior-posterior diameter of the chest. The chest should be allowed to recoil completely by releasing pressure so that the heart can refill. 20
Drug Support
Drugs are rarely needed in the delivery room resuscitation of the newborn if adequate ventilation has been established. If the heart rate continues below 60 beats/min despite adequate ventilation and compressions for a minimum of 30 seconds or if no heart rate is found, the transport team should instill 0.3 to 1 mL/kg of 1:10,000 solution of epinephrine down the endotracheal tube per protocol (Table 32-3). To ensure that the epinephrine reaches the lungs, it may be diluted in or flushed with 1 to 2 mL of normal saline solution for smaller volumes based on weight. Intravenous (IV) access should be attained as soon as possible, and the medication delivered intravenously rather than through the endotracheal tube. Current NRP guidelines for resuscitation in the delivery room recommend emergent low line placement into the umbilical vein as the most accessible parenteral route (Box 32-2). 20 If the heart rate remains below 60 beats/min, subsequent doses of epinephrine may be given every 3 to 5 minutes through the endotracheal tube or established IV line.
| IM, Intramuscular; UVC, umbilical venous catheter. | |||
| Drug | Indication | Dose | Route |
|---|---|---|---|
| Naloxone (neonatal Narcan) | Narcotic depression | 0.1 mg/kg | IV/UVC/IM/ETT |
| Epinephrine (1:10,000) | Bradycardia, cardiac arrest | 0.01 mg/kg – 0.03 mg/kg | ETT/UVC/IV |
| Sodium bicarbonate, 4.2% (0.5 mEq/mL) | Metabolic acidosis | 1-2 mEq/kg given slowly to reduce risk of intraventricular hemorrhage | UVC/IV (always clear line before and after administration) |
| 5% Albumin | Hypotension volume restoration | 10 mL/kg over 5-10 min | UVC/IV |
| Lactated Ringer’s | Hypotension volume restoration | 10 mL/kg over 5-10 min | UVC/IV |
| Dextrose 10% | Hypoglycemia | 2-4 mL/kg (list over 5 to 10 min) | UVC/IV |
BOX 32-2
Umbilical Vein Catheterization
The neonatal transport nurses should place the umbilical vein catheter with aseptic technique. The umbilical stump and surrounding skin should first be cleansed with an approved protocol that describes preparation of the area. For example, use alcohol first, then povidone-iodine (Betadine), and let the povidone-iodine dry for at least a minute; povidone-iodine can be wiped off when the procedure is completed. Drapes are then applied to create a sterile field. A 5F catheter is usually adequate and can normally be placed in all sizes of infants; it is prepared by either attaching the catheter directly to a three-way stopcock or trimming the flared end of the catheter and inserting a blunt needle adapter. The blunt needle adapter is then connected to the three-way stopcock and flushed with solution.
Umbilical tape is tied snugly around the base of the cord to provide control of bleeding during the procedure. The umbilical stump can be cut approximately 1 cm above the skin line. The neonatal transport nurse can then identify the two thick-walled constricted arteries (4-o’clock and 8-o’clock positions) and the thinner-walled larger vein (12-o’clock position). A pair of curved iris forceps may be helpful in identifying and opening the lumen of the vein.
The neonatal transport nurse then inserts the catheter tip in the lumen and gently advances it. Stabilizing the cord by gently holding the cord at the base or applying traction with a clamp on the Wharton’s jelly may be helpful. The umbilical vein normally runs in a cephalad direction. Directing the catheter in that line may assist in ease of entry. The venous catheter should be inserted only as far as necessary to obtain blood return, which is normally 2 to 3 cm. Further catheter advancement may result in a placement in the liver and consequent hepatic damage from medication injections.
The umbilical catheter can then be secured with a “goalpost” tape bridge. A purse-string suture around the Wharton’s jelly tied to the catheter controls bleeding and assists in securing the catheter.
Complications of this procedure include infection, hemorrhage, air emboli, and thrombus formation; therefore, the procedure should only be undertaken after appropriate training under supervision and then performed with extreme care.
Hypovolemia should be suspected with a history of bleeding or if the infant has poor response to resuscitation, pallor, or weak poorly palpable pulses that persist despite adequate ventilation. If hypovolemia is suspected, volume expanders should be given. As soon as possible, a blood sample should be evaluated for partial pressure of oxygen (PaO 2), partial pressure of carbon dioxide (PaCO 2), and pH. If an arterial sample is not available, a venous sample is still valuable, particularly for PaCO 2 and pH. 33
Maintenance of Body Temperature
As soon as possible after delivery, the infant should be dried, wet linens removed, and an external heat source provided, such as radiant warmers or double-walled isolette. If an external heat source is not immediately available, particular attention must be paid to ambient temperature, and the neonate should be swaddled in warm blankets with the head covered with a stocking cap if possible. The only circumstance in which thermoregulation efforts may be delayed is in the presence of meconium-stained amniotic fluid. These neonates should not be stimulated until the airway has been cleared with direct suctioning of the trachea to avoid the possibility of aspiration of any meconium that may be in the trachea.
Glucose Requirements
Neonates are susceptible to hypoglycemia because of immature glucose control mechanisms and decreased glucose substrate stores. The definition of hypoglycemia in the newborn may be variable depending on the reference. In actual practice, most clinicians consider a serum glucose of less than 40 mg/dL to represent hypoglycemia. 23,25 The infant who needs resuscitation or has conditions that necessitate transport to a higher level of care is generally at higher risk for hypoglycemia (Box 32-3). Infants identified as high risk should have screening glucose levels as soon as possible after delivery and every 30 minutes to 1 hour until normal serum levels have been achieved. Further discussion of glucose monitoring and stabilization is included in this chapter.
BOX 32-3
Risk Factors for Hypoglycemia
Small for gestational age (SGA)
Hypothermia or cold stress
Respiratory distress
Congenital heart disease
Large for gestational age (LGA)
Infant of a diabetic mother
Rh incompatibility
Beckwith-Wiedemann syndrome
Nesidioblastosis
Islet cell adenomas
Sepsis
Asphyxia
EVALUATION
If the neonate has not responded to the initial priorities of delivery room management, the transport team must reevaluate the clinical assessment and management of the infant. Common reasons for an inadequate response to resuscitation include the following:
1. Mechanical problems
a. Inadequate oxygen supply
b. Inadequate ventilation
2. Endotracheal tube malposition
a. Tube in esophagus
b. Tube in right or left mainstem bronchus
c. Obstructed tube
3. Unrecognized clinical problem
a. Congenital cardiac disease
b. Electrolyte imbalances
c. Congenital airway abnormality
d. Pneumothorax
e. Diaphragmatic hernia
NONINITIATION OR DISCONTINUATION OF RESUSCITATION
The International Guidelines for Neonatal Resuscitation for 2000 presents recommendations for the noninitiation or discontinuation of resuscitation, which include27,37:
▪ Birth weight less than 500 g.
▪ Confirmed trisomy 13 or 18.
▪ Congenital hydrocephalus.
▪ Gestational age less than 24 weeks.
▪ No response to ongoing adequate resuscitative efforts.
▪ Severe fetal growth retardation.
Each transport team should have policies and procedures that outline the management of these issues.
DETAILED ASSESSMENT AND ONGOING STABILIZATION
The goal of assessment and stabilization before transport is to ensure safe and optimal transport conditions for a neonate who has achieved or is being managed to support adequate vital signs, perfusion, optimal blood gases, and normal glucose and electrolyte levels. This goal is not always attainable, but every attempt should be made to maximize patient status and achieve stability before transport.
Assessment of the newborn should include history, clinical examination, and laboratory data. Obstetric information obtained should include the estimated day of confinement (EDC) based on the mother’s dates and clinical data, maternal age, gravity, parity, abortions, fetal demise, neonatal deaths, number of living children, length of rupture of membranes, and complications of the pregnancy, labor, or delivery. Maternal medications (both during pregnancy and during perinatal period), Group B streptococcus (GBS) status and associated treatment, any maternal infections, and any maternal illicit drug use should also be obtained. Neonatal history should include Apgar scores, resuscitation efforts, initial physical examination, and subsequent course. Laboratory data and radiographic studies should also be reviewed.
Gestational Age Examination
Basic knowledge of gestational age is helpful when considering the anatomy and physiologic immaturities of preterm infants. The transport team’s role in determination of the gestational age is obtaining a thorough maternal and delivery history. Most often the gestational age is determined before the transport team’s arrival with one of a variety of assessment tools. One example of an assessment tool is included in Figure 32-2.
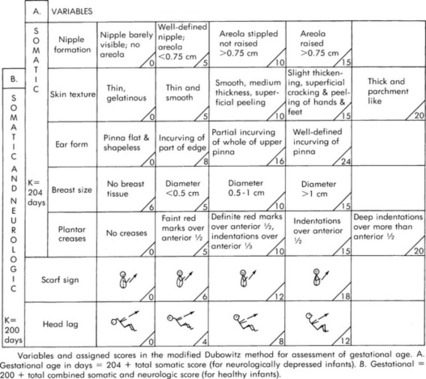 |
| FIGURE 32-2 Cappurro’s method for assessment of gestational age. (From Cappurro H, et al: A simplified method for diagnosis of gestational age in the newborn infant, J Pediatr 93:121, 1978.) |
Physical Examination
A considerable amount of baseline information can be obtained strictly through observation of the infant before disturbing the infant. This observation should include:
1. Heart: Rate, rhythm, heart sounds, murmurs, extra sounds.
2. Chest: Symmetry and adequacy of air entry, rales, rhonchi, wheezes.
3. Abdomen: Bowel sounds, organomegaly, masses.
4. Femoral pulses: Quality.
5. Signs and symptoms of distress: Color, respiratory effort, posture, and tone.
6. Obvious morphology.
Once the baseline examination has been established, the rest of the examination should proceed with an organized systematic approach. For example, the transport team might examine the infant beginning from the head and working downward. The essential components of a detailed examination are outlined as follows:
1. Head: Symmetry, shape, caput succedaneum, cephalhematoma.
2. Fontanelles/sutures: Fontanel number, fullness, depression, and size; suture mobility.
3. Symmetry of face: Development, shape, movement.
4. Ears: Shape, position of face, presence of skin tags.
5. Eyes: Shape, position, size, pupils, hemorrhages.
6. Mouth: Cleft palate, teeth, abnormalities, presence of micrognathia (in the event of the need for airway management).
7. Neck: Webbing, length.
8. Nose: Symmetry, septum, patency.
9. Clavicles: Masses, intactness.
10. Chest: Size, symmetry, shape.
11. Umbilical cord: Number of vessels.
12. Genitals: Development, testes, urethral and vaginal openings.
13. Anus: Patency, meconium.
14. Spine: Masses, symmetry, dimples.
15. Extremities: Symmetry, development, movement, pulses.
16. Hips: Range of motion.
17. Reflexes: Root, suck, Moro’s, grasp.
18. Tone: Flaccid, normal, jitteriness, flexion.
In the transport setting, the potential value of each part of the examination must be weighed against any stress it may cause to an already compromised infant and current physiologic stability.
Respiratory Management: General Considerations
The primary component of respiratory management is ensuring correct opening, positioning, and clearing of the airway. Because most ill neonates who need transport have some degree of respiratory compromise, the transport team must perform careful and continuous assessment of respiratory status. The presence of retractions, grunting, and nasal flaring may indicate a condition of decreased lung compliance. Both central and peripheral color should be evaluated for the presence of cyanosis. Adequacy of central oxygenation should be checked with evaluation of mucous membranes. The absence of central cyanosis typically indicates adequate pulmonary blood flow and gas exchange. Peripheral cyanosis, however, may reflect problems with either oxygenation or perfusion. If the infant is centrally oxygenated (pink) but peripherally cyanotic (purple), then the child should be evaluated to determine the cause of the poor perfusion. As a basic principle, sufficient oxygen should be supplied to ensure central oxygenation. Acrocyanosis is a self-limiting condition in which the neonate’s hands and feet and circumoral area may remain cyanotic for 24 to 48 hours after birth. Acrocyanosis may be a normal finding for the first 24 to 48 hours or it could suggest poor cardiac output, vasoconstriction, or cold stress; careful assessment, both physical and diagnostic, of fluid balance and environmental stressors may help identify causes of cyanosis.
Oxygen is a drug with risks and side effects associated with its use. Current technology, including use of pulse oximetry and end tidal carbon dioxide monitoring devices, greatly enhances the ability to titrate oxygen delivery based on patient response and need. Oxygen can be supplied with a number of methods: blow-by administration, head hoods or tents, nasal cannula, continuous positive airway pressure (CPAP), or endotracheal tube insertion. Blow-by or free-flow oxygen near the baby’s face can be used on a short-term basis but has two main drawbacks: first, accurate measurement of the exact percentage of oxygen that the baby is receiving is impossible; and second, the flow of cold oxygen into the nenoate’s face may result in increased inappropriate heat-generating responses and vagal stimulation. Hood oxygen allows for accurate measurement and stabilization of oxygen supply to the newborn but restricts accessibility to the newborn’s head without disturbing the oxygen concentration and is not practical for transport. Continuous positive airway pressure can be delivered via alternate modes that include nasal prongs, nasopharyngeal tube, and endotracheal tube. An endotracheal tube obviously provides the most effective delivery of CPAP, but it requires the invasive procedure of intubation. Positive-pressure ventilation requires the placement of an endotracheal tube, with its inherent potential complications, but it can be done safely in the hands of a skilled practitioner.
In selection of the mode of oxygen delivery, the transport team must weigh both the benefits to be gained and the risks incurred with the selected approach. Once respiratory support has begun, continuous observation and reevaluation must be accomplished to maintain the correct level of support. Adjustments must be made to accommodate changes in the infant’s pulmonary compliance. Diminishing compliance without an appropriate adjustment in oxygen support may result in hypoxia with hypercarbia. Improvement in compliance could result in hyperoxia, hypocarbia, and potentially air leaks with a resultant decrease in cardiac output. Any infant treated with positive pressure is at an automatic increased risk for air leaks, including pneumothorax, pneumomediastinum, and pulmonary interstitial emphysema. Uncommonly, pneumoperitoneum and pneumopericardium could also occur. Any sudden deterioration in an infant receiving positive-pressure ventilation should prompt immediate evaluation for a displaced endotracheal tube or pulmonary air leaks. Evaluation should include assessment of breath sounds, shifting of the point of maximal intensity (PMI), transillumination of the chest, and chest radiograph. 28 Mechanical problems with the oxygen delivery system should also be immediately ruled out. Excess air must always be removed from the stomach with a nasogastric or orogastric tube because a distended stomach could interfere with adequate ventilation.
Blood Pressure and Perfusion
Hypotension and poor perfusion are also common problems in the neonate. Assessment of circulatory status should begin with an evaluation of the obstetric history, which may suggest a cause for either hypovolemia or myocardial dysfunction. Historical facts suggestive of hypovolemia as a basis for poor perfusion may include compression of the cord or a history of blood loss during the pregnancy, labor, or delivery. Infants with a history of asphyxia may have both hypovolemia and myocardial dysfunction. Assessment should include arterial pressures, central venous pressures, extremity versus core temperatures, capillary refill greater than 3 seconds in the presence of a normal temperature, and evaluation of pulse volume. The presence of progressive metabolic acidosis in a well-ventilated and oxygenated infant and evidence of cardiomegaly on radiographic films may be indicative of an asphyxial cardiomyopathy.
Treatment is aimed at the return to adequate perfusion of the tissues to prevent continued metabolic acidosis. If the transport team suspects hypovolemia, treatment may include infusion of 10 mL/kg of an isotonic crystalloid solution such as normal saline solution. 20 Fluids should be infused slowly because a rapid increase in systemic blood pressure carries the risk of sudden rise in pressure in vascular beds, which could potentially cause capillary rupture and hemorrhage and possible intracranial bleeding. If myocardial dysfunction is suspected, the transport team may consider administration of inotropic agents per protocol. Whichever treatment is instituted, careful monitoring of arterial pressures is essential both to monitor results and to prevent complications. Neonates have limited ability to control their own cardiac output.
Thermoregulation
The neonate is at high risk for hypothermia because of large surface area compared with body mass and poor thermal insulation. Premature or hypoxic neonates are at increased risk for cold stress because of decreased stores of brown fat available for generation of heat production. 29 Hypothermia in the neonate is usually an iatrogenic condition, almost always preventable; if untreated, it can be contribute to neonatal death. A neonate can have a normal central temperature with axillary or skin temperature measurements and still be cold stressed with a cool skin temperature. Side effects of cold stress and hypothermia include increased oxygen consumption, hypoxemia, acidosis, and pulmonary vasoconstriction. In addition, the infant increases glucose consumption, which may result in hypoglycemia and increases the probability of mortality and morbidity.
The neonate maintains body temperature through basal metabolism, muscular activity, and chemical thermogenesis. The infant’s primary mechanism of heat production in response to cold stress is chemical thermogenesis with metabolizing brown fat stores. This process requires increased oxygen consumption and increased glucose utilization. 17 Compared with the adult, the neonate has a limited ability to produce heat by shivering, an increased oxygen consumption, and a limited ability to dissipate heat through sweating.
An understanding is essential of the mechanisms of heat production and loss and interventions for maintaining a neutral thermal environment during stabilization and transport. The neutral thermal environment is the range of environmental temperatures at which the neonate maintains a normal body temperature with minimal metabolic activity and oxygen consumption. 4,7,30,45
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree






