Edited by Mark Little Lindsay Murray Drug overdose in adults usually occurs in the context of self-poisoning, which may be either recreational or an act of deliberate self-harm. Deliberate self-poisoning accounts for 1–5% of all public hospital admissions in Australia [1,2]. The bulk of the medical management of cases presenting to hospital is carried out in the emergency department (ED) and the emergency physician is expected to be expert in the field. Although the management must vary considerably according to the nature and severity of the poisoning, some general principles apply. Above all, it must be remembered that the acute overdose presentation is only a discrete time-limited event in the course of the underlying condition, which is usually psychiatric or social in origin. The effects of ingestion of pharmaceuticals or illicit drugs range from the non-toxic to the life threatening and may involve any system. Poisoning is a dynamic presentation and the patient may present at varying points in the time course of the poisoning. Consequently, rapid clinical deterioration or improvement may be observed after the initial presentation and assessment. Acute morbidity and mortality from poisoning is usually a consequence of the cardiovascular, respiratory or central nervous system (CNS) complications of the poisoning. Less commonly, hepatic, renal or metabolic effects are potentially life threatening. The most frequent life-threatening respiratory complication of poisoning is ventilatory failure, which is usually a consequence of CNS depression. Less commonly, it is secondary to ventilatory muscle paralysis. The frequency and depth of respirations are reduced. Respiratory failure may also be caused by direct pulmonary toxicity or complications, such as pulmonary aspiration or non-cardiogenic pulmonary oedema (Table 29.1.1). Table 29.1.1 Toxic causes of respiratory failure Cardiovascular manifestations of poisoning include tachycardia, bradycardia, hypertension, hypotension, conduction defects and arrhythmias (Table 29.1.2). Bradycardia is relatively rarely observed and is associated with a number of potentially life-threatening ingestions. Tachycardia is commonly observed and is usually benign. It may be due to intrinsic sympathomimetic or anticholinergic effects of a drug or a reflex response to hypotension or hypoxia. Hypotension is also commonly observed and may be due to a number of different causes (Table 29.1.2). Hypertension is unusual. Severe hypertension is usually associated with illicit drug use and is important because it may produce complications such as intracerebral haemorrhage. Table 29.1.2 Cardiovascular effects of poisoning CNS manifestations of poisoning include decreased level of consciousness, agitation or delirium, seizures and disordered temperature regulation. A decreased level of consciousness is a common presentation of poisoning and is associated with many drugs, some of which are listed in Table 29.1.1. Although usually a direct drug effect, CNS depression is occasionally secondary to hypoglycaemia, hypoxia or hypotension. Common causes of agitation or delirium following overdose are listed in Table 29.1.3. Toxic seizures are potentially life threatening and important causes are listed in Table 29.1.4. Table 29.1.3 Toxic causes of agitation or delirium Table 29.1.4 Amphetamines Bupropion Carbamazepine Chloroquine Cocaine Isoniazid Mefanamic acid Theophylline Tramadol Tricyclic antidepressants Venlafaxine Hypothermia is usually a complication of environmental exposure secondary to a decreased level of consciousness or altered behaviour. Hyperthermia is a direct toxic effect and causes are listed in Table 29.1.5. Severe hyperthermia is rapidly lethal if not corrected. Table 29.1.5 Amphetamines Anticholinergics Cocaine MAO inhibitors Salicylates Serotonin syndrome Metabolic and other manifestations of poisoning include hyper- and hypoglycaemia, hyper- and hyponatraemia, acidosis and alkalosis and hepatic failure. Acute poisoning is distinguished from many other forms of acute illness in that, given appropriate supportive care over a relatively short period, a full recovery can usually be expected. A small number of potentially fatal poisonings may demonstrate progressive toxicity despite full supportive care. These are the so-called cellular toxins and include colchicine, iron, salicylate, cyanide, paracetamol, theophylline and digoxin. In some of these cases, early aggressive gastrointestinal decontamination, timely administration of antidotes or the institution of techniques of enhanced elimination may be life saving. Mortality or morbidity may also result from specific complications of a poisoning. These include trauma, pulmonary aspiration, adult respiratory distress syndrome, rhabdomyolysis, renal failure and hypoxic encephalopathy. These complications usually occur prior to arrival in the ED. Pulmonary aspiration frequently complicates a period of decreased level of consciousness or a seizure. It is a leading cause of in-hospital morbidity and mortality following overdose. This complication is characterized by rapid onset of dyspnoea, cough, fever, wheeze and cyanosis. Rhabdomyolysis occurs as a direct toxic effect (rare) or secondary to excessive muscular hyperactivity, seizures, hyperthermia or prolonged coma with direct muscle compression. The urine is dark and acute renal failure can develop secondary to tubular deposition of myoglobin. A risk assessment should be made as soon as possible in the management of the poisoned patient. Only resuscitation is a greater priority (Table 29.1.6). Risk assessment is a distinct quantitative cognitive step through which the clinician attempts to predict the likely clinical course and potential complications for the individual patient at that particular presentation [3]. An accurate risk assessment allows informed decision making in regard to all subsequent management steps including duration and intensity of supportive care and monitoring, screening and specialized testing, decontamination, enhanced elimination, antidotes and disposition. Factors that are taken into account when formulating this risk assessment include: the agent(s), the dose, the time since ingestion, the clinical features present and patient factors (Table 29.1.6). Specialized testing may refine risk assessment. Access to specialized poisons information in the form of a poisons information centre or in-house databases is often necessary to formulate an accurate risk assessment. Table 29.1.6 Risk assessment-based approach to poisoning Reproduced from Murray L, Daly F, Little M, Cadogan M. Toxicology handbook, 2nd edn. Sydney: Elsevier; 2011. Every effort should be made to obtain information as to the type and dose of drug ingested, the time of ingestion and the progression of symptoms since ingestion. History provided by the patient, if they are awake, is usually reliable and should not be dismissed. The focused physical examination of the poisoned patient aims to: The initial physical examination of the overdose patient in many ways parallels the primary survey of the trauma patient. The airway, breathing and circulation are assessed and stabilized as necessary. The level of consciousness should be assessed, the presence of seizure activity noted and the blood glucose and temperature measured. A more complete examination is carried out when the patient is stable. This should include a full neurological examination, including assessment of the level of consciousness and mental status, pupil size, muscle tone and movements and the presence or absence of focal neurological signs. Poisoning normally causes global CNS depression and focal signs suggest an alternative diagnosis or a CNS complication, such as cerebral haemorrhage. Other features that should be specifically sought are any evidence of associated trauma, the state of hydration, the condition of the skin, in particular the presence of pressure areas, the presence or absence of bowel sounds and the condition of the urine. Several toxic autonomic syndromes, or ‘toxidromes’, have been described in relation to poisoning. The principal ones are listed in Table 29.1.7. Identification of these syndromes may narrow the differential diagnosis in cases of unknown poisoning. Table 29.1.7 Toxic autonomic syndromes or ‘toxidromes’ NSSRI: non-selective serotonin re-uptake inhibitor; SSRI: selective serotonin re-uptake inhibitor. Information on the clinical course and toxic doses of specific pharmaceutical and non-pharmaceutical poisons is available on a 24 h basis throughout Australia by telephoning 131126. The poison information centres are staffed by trained poisons information specialists and are also able to refer cases to clinical toxicologists for consultation. The management of poisoning should be approached in a systematic way. Following initial resuscitation, further treatment is informed by the risk assessment (see Table 29.1.6). Supportive care is the key element in the management of poisoning. The vast majority of poisonings result in temporary dysfunction of one or more of the body systems. If appropriate support of the system in question is instituted in a timely fashion and continued until the toxic substance is metabolized or excreted, a good outcome can be anticipated. In severe poisonings, supportive care may be very aggressive and possible interventions are listed in Table 29.1.8. Table 29.1.8 Supportive care measures for the poisoned patient The specific supportive management of a number of manifestations or complications of poisoning warrants further mention insofar as it may differ from the standard management of such conditions with other aetiologies. Cardiopulmonary arrest from poisoning should be aggressively resuscitated. Direct current cardioversion is rarely successful in terminating toxic arrhythmias and should not take precedence over establishing adequate ventilation and oxygenation, cardiac compressions, correction of acidosis or hypovolaemia and the administration of specific antidotes. Resuscitative efforts should be continued beyond the usual time frame. In cardiac arrest due to drugs with direct cardiac toxicity, the use of cardiopulmonary bypass or extracorporeal membrane oxygenation (ECMO) until the drug is metabolized may be life saving. In general, intravenous benzodiazepines are the drugs of choice for control of toxic seizures. Large doses may be required. Hypoxia and hypoglycaemia must be corrected if they are contributory factors. Patients with toxic seizures do not generally need long-term anticonvulsant therapy. Isoniazid-induced seizures are difficult to control without administration of an adequate dose of the specific antidote, pyridoxine. The management of pulmonary aspiration is essentially supportive, with supplemental oxygenation and intubation and mechanical ventilation if necessary. Neither prophylactic antibiotics nor corticosteroids have been shown to be helpful in the management of this condition, which is essentially a chemical pneumonitis. Toxic hypertension rarely requires specific therapy. Most cases are mild and simple observation is sufficient. Agitation or delirium is a feature of many intoxications associated with hypertension and adequate sedation with benzodiazepines usually lowers the blood pressure. Severe toxic hypertension is most likely in toxicity from cocaine or amphetamine-type drugs and treatment may be indicated to avoid complications, such as cardiac failure or intracerebral haemorrhage. The drug of choice in this situation is sodium nitroprusside by intravenous infusion. The extremely short duration of action of this vasodilator allows accurate control of hypertension during the toxic phase and avoids the development of hypotension once toxicity begins to wear off. Management of rhabdomyolysis consists of treatment of the causative factors, fluid resuscitation and careful monitoring of fluids and electrolytes. The role of mannitol and urinary alkalinization in reducing the risk of renal failure is not clear. Established acute renal failure requires haemodialysis, often for up to 6 weeks. The aim of decontamination of the gastrointestinal tract is to bind or remove ingested material before it is absorbed into the circulation and able to exert its toxic effects. This is a very attractive concept and has long been considered one of the fundamental interventions in management of the overdose patient. However, gastrointestinal decontamination should not be regarded as a routine procedure in the management of the patient presenting to the ED following an overdose. The decision to perform gastrointestinal decontamination and the choice of method should be based on an assessment of the likely benefit, the likely risk and the resources required. Gastrointestinal decontamination should only be considered where there is likely to be a significant amount of a significantly toxic material remaining in the gut. It is never indicated when the risk assessment predicts a benign course. Efforts at decontamination technique should never take precedence over the institution of appropriate supportive care. Three basic approaches to gastrointestinal decontamination are available: gastric emptying, administration of an adsorbent and catharsis. Gastric emptying can be attempted by the administration of an emetic, most commonly syrup of ipecac, or by gastric lavage. In volunteer studies, both of these techniques removed highly variable amounts of marker substances from the stomach even if performed immediately after ingestion and the effect diminished rapidly with time to the point of being negligible after 1 hour [4,5]. Clinical outcome trials have failed to demonstrate improved outcome as a result of routine gastric emptying in addition to administration of activated charcoal, except, perhaps, in patients presenting unconscious within 1 h of ingestion [6–8]. The principal adsorbent available to clinicians is activated charcoal (AC), which effectively binds most pharmaceuticals and chemicals, and is currently the decontamination method of choice for most poisonings. Materials that do not bind well to charcoal are listed in Table 29.1.9. Table 29.1.9 Materials that do not bind well to activated charcoal Charcoal is ‘activated’ by treatment in acid and steam at high temperature. This process removes impurities and greatly increases the surface area available for binding. Activated charcoal (AC) is packaged as a 50 g dose premixed with water or sorbitol, which is likely to be sufficient for the majority of ingestions. Adult patients are usually able to drink AC slurry from a cup. If the level of consciousness is too impaired to allow this, they should be intubated first. Administration of AC is absolutely contraindicated unless the patient has an intact or protected airway. Volunteer studies demonstrate that the effect of AC diminishes rapidly with time and that the greatest benefit occurs if it is administered within 1 h. There is as yet no evidence that AC improves clinical outcome [9]. There is no evidence to suggest that the addition of a cathartic, such as sorbitol, to AC improves clinical outcome [10]. Apart from rarely employed endoscopic and surgical techniques, whole-bowel irrigation (WBI) is the most aggressive form of gastrointestinal decontamination. Polyethylene glycol solution (Golytely is administered via a nasogastric tube at a rate of 2 L/h until a clear rectal effluent is produced. This usually takes about 6 h and requires one-to-one nursing. In volunteer studies, this technique reduced the absorption of slow-release pharmaceuticals and so may be of benefit in life-threatening overdoses of these agents. Again, clinical benefit has not yet been conclusively demonstrated [11]. The use of WBI has also been reported in the management of potentially toxic ingestions of iron, lead and packets of illicit drugs. Whole-bowel irrigation is contraindicated if there is evidence of ileus or bowel obstruction and in patients who have an unprotected airway or haemodynamic compromise. A number of techniques are available to enhance the elimination of toxins from the body. Their use is rarely indicated, as only a very few drugs capable of causing severe poisoning have pharmacokinetic parameters that render them amenable to these techniques (Table 29.1.10). Table 29.1.10 Techniques of enhanced elimination Multiple-dose AC (25–50 g every 3–4 h) may enhance drug elimination by interrupting the enterohepatic circulation or by ‘gastrointestinal dialysis’. Gastrointestinal dialysis is the movement of a toxin across the gastrointestinal wall from the circulation into the gut down a concentration gradient that is maintained by charcoal binding. For this technique to be effective, a drug must undergo considerable enterohepatic circulation or, in the case of ‘gastrointestinal dialysis’, have a small volume of distribution, small molecular weight, low protein binding, slow endogenous elimination and bind to charcoal [12]. The advantages of this technique are that it is non-invasive and simple to carry out. Alkalinization of the urine enhances urinary excretion of drugs that are filtered at the glomerulus and are unable to be reabsorbed across the tubular epithelium when in an ionized form at alkaline pH. For elimination to be effectively enhanced by this method, the drug must be predominantly eliminated by the kidneys in the unchanged form, have a low pKa, be distributed mainly to the extracellular fluid compartment and be minimally protein bound. Haemodialysis (HD) and haemoperfusion (HP) are both very invasive techniques and for that reason are reserved for potentially life-threatening intoxications. Only a small number of drugs that have small volumes of distribution, slow endogenous clearance rates, small molecular weights (HD) and bind to charcoal (HP) will have their rates of elimination significantly enhanced by these procedures. Very few drugs have effective antidotes. Occasionally, however, timely use of an antidote may be life saving or substantially reduce morbidity, time in hospital or resource requirements. Antidotes that may be indicated in the ED setting are listed in Table 29.1.11. However, it must be remembered that antidotes are also drugs and are frequently associated with adverse effects of their own. An antidote should only be used where a specific indication exists and then only at the correct dose, by the correct route and with appropriate monitoring. Because many antidotes are so infrequently used, obtaining sufficient supplies when the need arises can be difficult. Every ED must review its stocking of antidotes and have a plan for obtaining further supplies should the need arise. Table 29.1.11 It is essential to exclude important non-toxic diagnoses in the patient presenting with coma or altered mental status presumed to be due to drug overdose. These diagnoses include head injury, intracerebral haemorrhage or infarction, CNS infection, hyponatraemia, hypoglycaemia, hypo- or hyperthermia, post-ictal states and psychiatric disorders. Investigations should only be performed if they are likely to affect the management of the patient. They are employed as either screening tests or for specific purposes. In poisoning, screening tests aim to identify occult toxic ingestions for which early specific treatment might improve outcome. The recommended screening tests for acute poisoning are the 12-lead ECG and the serum paracetamol level. The ECG is used to exclude conduction defects which may predict potentially life-threatening cardiotoxicity. The serum paracetamol is useful to ensure that paracetamol poisoning is diagnosed within the time available for effective antidotal treatment. Other specific investigations may be indicated to exclude important differential diagnoses, confirm a specific poisoning for which significant complications might be anticipated, assess the severity of intoxication, assess response to treatment or assess the need for a specific antidote or enhanced elimination technique. The patient with only minor manifestations of poisoning may require no other blood tests apart from a screening paracetamol level. Pregnancy should be excluded in women of childbearing age by serum or urine β-HCG if necessary. More seriously ill patients may require electrolyte, renal and liver function tests and a full blood count, creatine kinase and arterial blood gases. Urinalysis reveals myoglobinuria in significant rhabdomyolysis. Routine qualitative drug screening of urine or blood in the overdose patient is rarely useful in planning management. Measurement of serum drug concentrations is only useful if this provides important diagnostic or prognostic information or assists in planning management. Some drug levels that may be useful are listed in Table 29.1.12. For most cases, drug overdose management is guided by clinical findings and not by drug levels. Some drugs commonly taken in overdose for which serum concentrations are of no value in planning management are listed in Table 29.1.13. Table 29.1.12 Drug levels that may be helpful in the management of selected cases of overdose Carbamazepine Digoxin Dilantin Lithium Iron Paracetamol Phenobarbitone Salicylate Theophylline Valproate Table 29.1.13 Drug levels that are not helpful in the management of overdose Radiology has a limited role in the management of overdose. A chest X-ray is indicated in any patient with a significantly decreased level of consciousness, seizures or hypoxia. It may show evidence of pulmonary aspiration. A computed tomography scan of the head may be indicated to exclude other intracranial pathology in the patient with an altered mental status. The abdominal X-ray is useful in evaluating overdose of radiopaque metals including iron, lithium, potassium, lead and arsenic. Both the medical and the psychiatric disposition of the overdose patient must be considered. A good risk assessment is essential to determining timely and safe disposition. The majority of overdose patients who remain stable at 4–6 h after the ingestion do not need further close monitoring and may be admitted to a non-monitored bed until manifestations of toxicity completely resolve. An emergency observation ward is ideal for this purpose. Any patient who develops clinical manifestations of intoxication severe enough to require the institution of specific supportive care measures requires admission to an intensive care environment. A few patients will require admission for prolonged monitoring based on the history of the ingestion. For example, anyone with a history of ingestion of colchicine, organophosphates, slow-release theophylline or slow-release calcium channel blockers requires admission because of the possibility of delayed onset of severe toxicity. Psychiatric evaluation of deliberate self- poisoning cases is indicated as soon as the patient’s medical condition permits. All such patients must be continuously supervised until the psychiatric evaluation has taken place. Betty Shuk Han Chan and Angela Chiew The calcium channel blockers (CCBs) and β-blockers are widely prescribed in the community. In overdose, they present with similar clinical pictures of potentially life-threatening impairment of cardiac function. The management of both types of overdose is similar and they are discussed together. Standard CCB preparations are rapidly absorbed from the gastrointestinal tract, with onset of action occurring within 30 min. Pharmacokinetic parameters are shown in Table 29.2.1. Verapamil and diltiazem undergo significant first-pass hepatic clearance. Verapamil is metabolized to norverapamil, which possesses 15–20% of verapamil’s pharmacological activity and is renally excreted. Diltiazem is metabolized to deacetyldiltiazem, which has half the potency of the parent compound and undergoes biliary excretion. The elimination half-lives of all CCBs may be prolonged following massive overdose. Amlodipine has a longer plasma half-life (30–50 h) than other CCBs. Table 29.2.1 Pharmacological profiles of the calcium channel blockers
Toxicology Emergencies
29.1 Approach to the poisoned patient
Introduction
Pathophysiology and clinical features



Assessment
Risk assessment
Resuscitation
Risk assessment
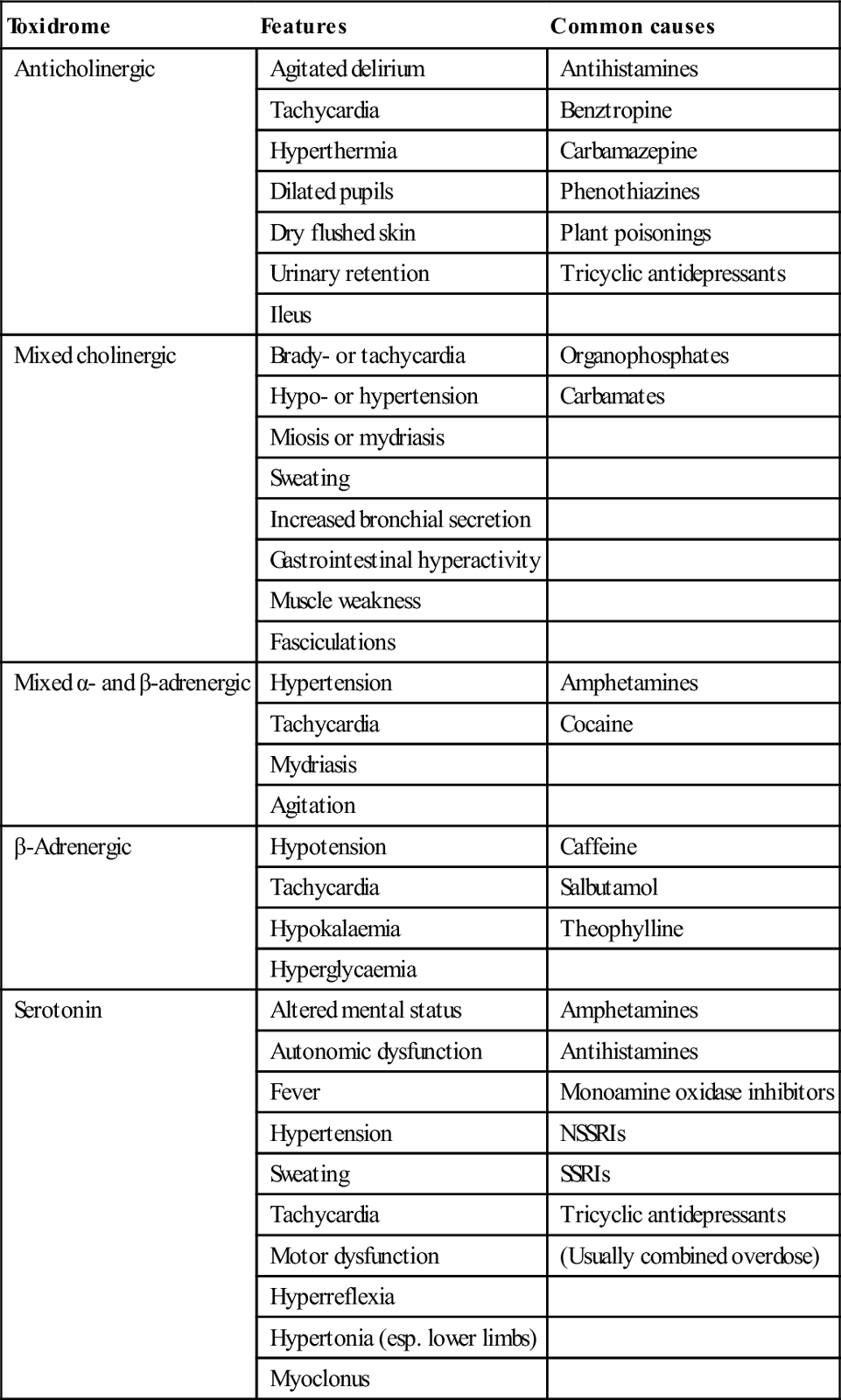
Supportive care and monitoring
Investigations
Decontamination
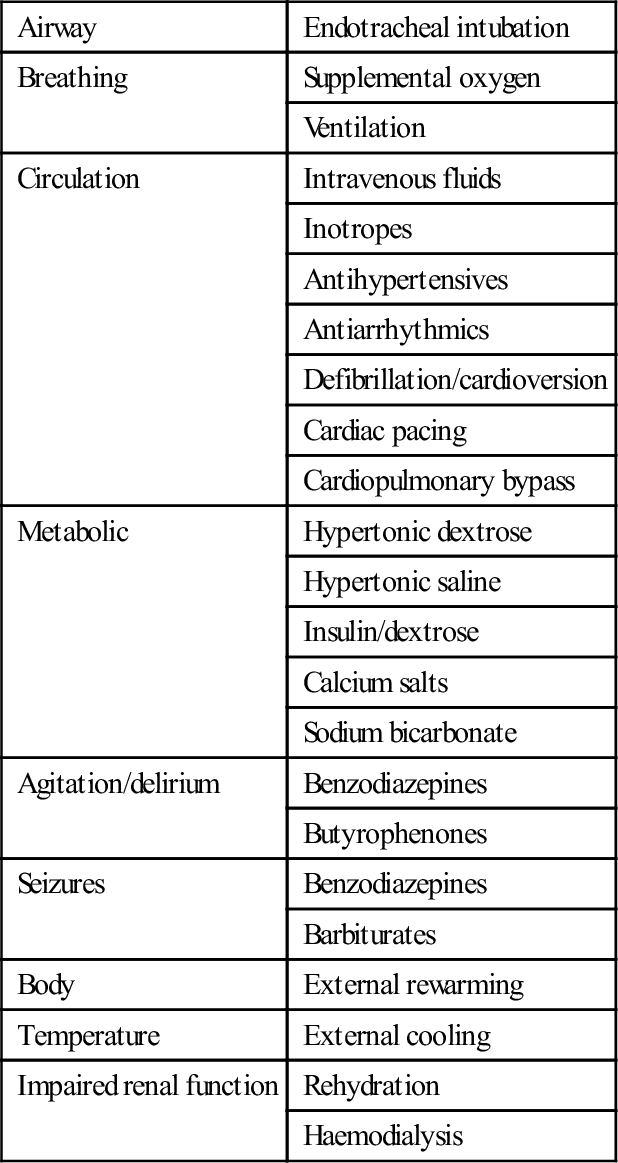
Enhanced elimination
Antidotes
Disposition

History
Physical examination
 identify any immediate threats to life and the need for intervention
identify any immediate threats to life and the need for intervention
 establish a baseline clinical status
establish a baseline clinical status
 identify intoxication syndromes
identify intoxication syndromes
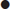 identify possible alternative diagnoses
identify possible alternative diagnoses
Toxidrome
Features
Common causes
Anticholinergic
Agitated delirium
Antihistamines
Tachycardia
Benztropine
Hyperthermia
Carbamazepine
Dilated pupils
Phenothiazines
Dry flushed skin
Plant poisonings
Urinary retention
Tricyclic antidepressants
Ileus
Mixed cholinergic
Brady- or tachycardia
Organophosphates
Hypo- or hypertension
Carbamates
Miosis or mydriasis
Sweating
Increased bronchial secretion
Gastrointestinal hyperactivity
Muscle weakness
Fasciculations
Mixed α- and β-adrenergic
Hypertension
Amphetamines
Tachycardia
Cocaine
Mydriasis
Agitation
β-Adrenergic
Hypotension
Caffeine
Tachycardia
Salbutamol
Hypokalaemia
Theophylline
Hyperglycaemia
Serotonin
Altered mental status
Amphetamines
Autonomic dysfunction
Antihistamines
Fever
Monoamine oxidase inhibitors
Hypertension
NSSRIs
Sweating
SSRIs
Tachycardia
Tricyclic antidepressants
Motor dysfunction
(Usually combined overdose)
Hyperreflexia
Hypertonia (esp. lower limbs)
Myoclonus
Poisons information
Treatment
Resuscitation, supportive care and monitoring
Airway
Endotracheal intubation
Breathing
Supplemental oxygen
Ventilation
Circulation
Intravenous fluids
Inotropes
Antihypertensives
Antiarrhythmics
Defibrillation/cardioversion
Cardiac pacing
Cardiopulmonary bypass
Metabolic
Hypertonic dextrose
Hypertonic saline
Insulin/dextrose
Calcium salts
Sodium bicarbonate
Agitation/delirium
Benzodiazepines
Butyrophenones
Seizures
Benzodiazepines
Barbiturates
Body
External rewarming
Temperature
External cooling
Impaired renal function
Rehydration
Haemodialysis
Decontamination

Enhanced elimination
Technique
Suitable toxin
Multiple-dose activated charcoal
Carbamazepine
Dapsone
Phenobarbitone
Phenytoin
Theophylline
Urinary alkalinization
Phenobarbitone
Salicylate
Haemodialysis
Ethylene glycol
Lithium
Metformin lactic acidosis
Methanol
Salicylate
Theophylline
Valproic acid
Charcoal haemoperfusion
Theophylline
Antidotes
Poisoning
Antidote
Atropine
Physostigmine
Benzodiazepines
Flumazenil
Cyanide
Dicobalt edetate, hydroxocobalamin
Digoxin
Digoxin-specific
Fab fragments
Insulin
Dextrose
Iron
Desferoxamine
Isoniazid
Pyridoxine
Methaemoglobinaemia
Methylene blue
Methanol and ethylene glycol
Ethanol, fomepizole
Organophosphates and carbamates
Atropine, oximes
Opioids
Naloxone
Paracetamol
N-acetyl cysteine
Sulphonylureas
Dextrose, octreotide
Tricyclic antidepressants
Sodium bicarbonate
Warfarin, brodifacoum
Vitamin K
Differential diagnosis
Clinical investigations
CNS drugs
Cardiovascular drugs
Antidepressants
ACE inhibitors
Benzodiazepines
β-Blockers
Benztropine
Calcium channel blockers
Cocaine
Clonidine
Newer antipsychotics
Opiates
Phenothiazines
Disposition
29.2 Cardiovascular drugs
Calcium channel blockers and β-blockers
Introduction
Pharmacokinetics
Class
Phenylalkylamines
Benzothiazepines
Dihydropyridines
Prototype
Verapamil
Diltiazem
Nifedipine
Hours to peak plasma concentration (NR/SR)
1.5/5–7
2.3/5–11
0.5/5
Half-life (h)
3–7/10–12
3–5/6–7
2–5–5–7
Half-life in massive overdose (h)
10–12
8–9
7–8
Absorption (%)
>90
>90
>90
Vd (L/kg)
4
5
1.2
Protein binding (%)
90
80–90
90
Predominant excretion route
(1) Hepatic; (2) renal
Hepatic
Renal
Active metabolite
Yes (20%)
Yes (25–50%)
No
Heart rate (%)
−10
−15
+10
Systemic vascular resistance (%)
−10
−10
−20
AV node conduction velocity (%)
−20
−25
+10 
Full access? Get Clinical Tree


29. Toxicology Emergencies
Only gold members can continue reading. Log In or Register to continue