FIGURE 26.1 Pressure and flow changes during a single ventilator breath using volume control and pressure control methods of lung inflation at equivalent inflation (tidal) volumes. Changes in airway pressure (Paw) are indicated by the solid lines, and changes in alveolar pressure (Palv) are indicated by the dashed lines. I = inspiration, E = expiration.
Volume Control
With volume control ventilation (VCV), the inflation (tidal) volume is preselected, and the lungs are inflated at a constant flow rate until the desired volume is delivered. Because there is airflow at the end of inspiration, the peak pressure in the proximal airways (peak Paw) is greater than the peak pressure in the alveoli (peak Palv); and the difference (peak Paw – peak Palv) is the pressure dissipated by the resistance to flow in the airways. The peak alveolar pressure is a reflection of the alveolar volume at the end of lung inflation.
Advantages
CONSTANT TIDAL VOLUME: The major advantage of VCV is the ability to deliver a constant tidal volume despite changes in the mechanical properties of the lungs. When airways resistance increases or lung compliance decreases, the ventilator generates a higher pressure to deliver the preselected volume. This maintains the desired minute ventilation in the face of abrupt or undetected changes in airway resistance or lung compliance.
Disadvantages
AIRWAY PRESSURE: At any given tidal volume, the airway pressures at the end of inspiration are higher during VCV than during pressure control ventilation (PCV), as shown in Figure 26.1, and this is mistakenly perceived as an increased risk of ventilator-induced lung injury. However, the risk of ventilator-induced lung injury is related to the peak alveolar pressure (2,4), and this pressure is the same during VCV and PCV (at equivalent tidal volumes). Therefore, the higher peak airway pressures during volume-controlled ventilation do not increase the risk of alveolar overdistension and ventilator-induced lung injury.
INSPIRATORY FLOW: There are disadvantages related to the constant inspiratory flow rate during VCV. First, the duration of inspiration is relatively short, and this can lead to uneven alveolar filling. In addition, the maximum inspiratory flow is limited when flow is constant, and the inspiratory flow rate can be inadequate in patients with high flow demands, resulting in patient distress. A decelerating flow pattern is available for VCV, and it has been shown to improve patient comfort VCV (5).
Pressure Control
With pressure control ventilation (PCV), the desired inflation pressure is preselected, and a decelerating inspiratory flow rate provides high flows at the onset of the lung inflation, to attain the desired inflation pressure quickly. The inspiratory time is adjusted to allow enough time for the inspiratory flow rate to fall to zero at the end of inspiration. Since there is no airflow at the end of the inspiration, the end-inspiratory airway pressure is equivalent to the peak alveolar pressure (see Figure 26.1).
Advantages
ALVEOLAR PRESSURE: The major benefit of PCV is the ability to control the peak alveolar pressure, which is the pressure most closely related to the risk of alveolar overdistension and ventilator-induced lung injury. Clinical studies indicate that the risk of ventilator-induced lung injury is negligible if the peak alveolar pressure is ≤30 cm H2O (2,4 ). (Note: Maintaining a peak alveolar pressure ≤30 cm H2O is also possible during VCV by monitoring the end-inspiratory occlusion pressure or “plateau pressure,” which is described on pages 489–490.)
PATIENT COMFORT: PCV is more likely to promote patient comfort than VCV (6), and this has been attributed to the high initial flow rates and the longer duration of inspiration with PCV.
Disadvantages
ALVEOLAR VOLUME: The major disadvantage of PCV is the decrease in alveolar volume that occurs when there is an increase in airway resistance or a decrease in lung compliance. This is a particular concern in acute respiratory failure, where steady-state conditions of airway resistance and lung compliance are unlikely.
Pressure-Regulated, Volume Control
Pressure-regulated, volume control ventilation (PRVC) is a hybrid mode of ventilation that provides a constant tidal volume (like volume control) but limits the end-inspiratory airway pressures (like pressure control). PRVC operates like an intelligent form of volume control; i.e., the ventilator monitors lung compliance, and uses these measurements to select the lowest airway pressure needed to deliver the desired tidal volume. Hybrid modes like PRVC are gaining in popularity, although there is no documented clinical advantage with PRVC when compared to more conventional modes of ventilation.
ASSIST-CONTROL VENTILATION
Assist-control ventilation (ACV) allows the patient to initiate a ventilator breath (assisted or patient-triggered ventilation), but if this is not possible, ventilator breaths are delivered at a preselected rate (controlled or time-triggered ventilation). The ventilator breaths during ACV can be volume-controlled or pressure-controlled.
Triggers
ACV involves two types of ventilator breaths, which are illustrated in the upper panel of Figure 26.2. The pressure waveform on the left is preceded by a negative pressure deflection, which represents a spontaneous inspiratory effort by the patient. This is a patient-triggered ventilator breath. The pressure waveform on the right is not preceded by a negative pressure deflection, indicating the absence of a spontaneous inspiratory effort. In this case, there is no interaction between the patient and the ventilator, and the ventilator breaths are delivered at a preselected rate. This is a time-triggered ventilator breath.
Patient-Related Triggers
There are two types of signals used for patient-triggered breaths: negative pressure and inspiratory flow rate.
NEGATIVE PRESSURE: Patients can trigger a ventilator breath by generating a negative airway pressure of 2 to 3 cm H2O, which opens a pressure-sensitive valve in the ventilator. This is double the negative airway pressure generated during quiet breathing (7), which may explain why one-third of inspiratory efforts fail to trigger a ventilator breath when negative pressure is the trigger signal (8).
FLOW RATE: Flow triggering involves little or no change in pressure and volume, and thus involves less mechanical work than pressure triggering (9). For this reason, flow has replaced pressure as the standard trigger mechanism. The flow rate that is required to trigger a ventilator breath differs for each brand of ventilator, but rates of 1–10L/min are usually required. Auto-triggering from system leaks (which create flow changes) is the major problem associated with flow triggering.
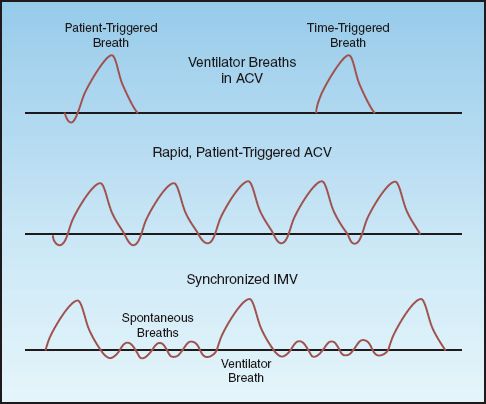
FIGURE 26.2 Airway pressure patterns in assist-control ventilation (ACV) and synchronized intermittent mandatory ventilation (SIMV). See text for further explanation.
The Respiratory Cycle
A general rule of thumb during mechanical ventilation is to allow at least twice the amount of time for expiration as allowed for inspiration. This is equivalent to an inspiration:expiration time ratio (I:E ratio) of at least 1:2. The goal is to allow enough time for complete exhalation to prevent dynamic hyperinflation and intrinsic or occult PEEP (see page 491). If the duration of exhalation is too short, the I:E ratio can be increased by: (a) increasing the inspiratory flow rate, (b) reducing the tidal volume, or (c) decreasing the inspiratory time (for pressure control).
Rapid Breathing
When each breath is a patient-triggered ventilator breath, rapid breathing like that shown in Figure 26.2 can severely curtail the time for exhalation and increase the risk of incomplete alveolar emptying and intrinsic PEEP. When rapid breathing is the result of a condition other than discomfort or anxiety, attempts to reduce the respiratory rate with sedation or inspiratory flow adjustments are often unsuccessful. In this situation, the appropriate mode of ventilation is described next.
INTERMITTENT MANDATORY VENTILATION
The Method
Difficulties with rapid breathing during ACV in neonates with respiratory distress syndrome, who typically have respiratory rates above 40 breaths/minute, led to the introduction of intermittent mandatory ventilation (IMV). IMV is designed to allow spontaneous breathing between ventilator breaths. (Spontaneous breathing is the condition where the patient initiates and terminates each lung inflation.) This is accomplished by placing a spontaneous breathing circuit in parallel with the ventilator circuit with a unidirectional valve that opens the spontaneous breathing circuit when a ventilator breath is not being delivered. The ventilatory pattern for IMV is illustrated in the lower panel of Figure 26.2. Note that the ventilator breath is delivered in synchrony with the spontaneous breath; this is called synchronized IMV (SIMV).
The ventilator breaths during SIMV can be volume-controlled or pressure-controlled. The rate of ventilator breaths is adjusted as needed to match the total minute ventilation (spontaneous plus assisted breaths) to the patient’s baseline level.
Adverse Effects
The principal adverse effects of IMV are: (a) an increased work of breathing, and (b) a decrease in cardiac output, primarily in patients with left ventricular dysfunction. Both effects are the result of the spontaneous breathing period.
Work of Breathing
The heightened work of breathing during the spontaneous breathing period in IMV is attributed to resistance in the ventilator circuit, which can have an exaggerated effect on ventilatory work when breathing is rapid (the condition that usually prompts the use of IMV). Pressure-support ventilation (described in the next section) overcomes the added resistance of the ventilator circuit, and thereby reduces the work of breathing (10). As a result, pressure-support ventilation (at 10 cm H2O) is now used routinely during the spontaneous breathing periods in IMV.
Cardiac Output
As described at the end of the last chapter, positive pressure ventilation reduces left ventricular afterload and increases cardiac output, primarily in patients with left ventricular dysfunction (see Figure 25.7 on page 501) (11). IMV has the opposite effect and increases left ventricular afterload (during the spontaneous breathing periods), which results in a decrease in cardiac output in patients with left ventricular dysfunction (12).
Summary
The major indication for IMV is rapid breathing with incomplete exhalation during assist-control ventilation. The spontaneous breathing periods during IMV promote alveolar emptying and reduce the risk of air trapping and intrinsic PEEP. IMV can increase the work of breathing and impair cardiac output in patients with left ventricular dysfunction; as a result, IMV is not advised for patients with respiratory muscle weakness or left heart failure.
PRESSURE SUPPORT VENTILATION
Pressure support ventilation (PSV) is pressure-augmented spontaneous breathing (13). It differs from patient-triggered PCV because it allows the patient to terminate the lung inflation, whereas the ventilator terminates the lung inflation during patient-triggered PCV. Therefore, PSV is a more interactive form of ventilation than PCV because it allows the patient to control the inspiratory time and tidal volume.
The Pressure-Supported Breath
The changes in pressure, and flow during a lung inflation with PSV are shown in Figure 26.3. PSV uses a decelerating inspiratory flow rate, with high flow rates early in inspiration to achieve the desired pressure level. The pressure-augmented breath is terminated when the inspiratory flow rate falls to 25% of the peak level. This allows the patient to determine the duration of lung inflation and the tidal volume.
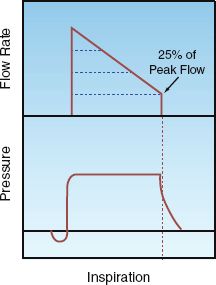
FIGURE 26.3 Changes in pressure and flow during a single lung inflation with pressure support ventilation. The lung inflation is terminated when the inspiratory flow rate falls to 25% of the peak flow rate, which allows the patient to determine the inspiratory time and tidal volume.
Clinical Uses
Low levels of PSV (5–10 cm H2O) can be used during weaning from mechanical ventilation, to overcome the resistance to flow in the artificial airways and ventilator tubing. The goal of PSV in this setting is to reduce the work of breathing without augmenting the tidal volume (14). Higher levels of PSV (15–30 cm H2O) can be used to augment the tidal volume; in this case, PSV is used as a form of noninvasive ventilation (which is described in the next chapter) (15).
POSITIVE END-EXPIRATORY PRESSURE
Rationale
Progressive narrowing of the airways during expiration can culminate in collapse of distal airspaces (small airways and alveoli) at the end of expiration. This normally occurs in dependent lung regions, where transpulmonary pressures are more positive (gravitational effect). The transpulmonary pressure where the distal airspaces begin to collapse, is called the closing pressure, and is normally about 3 cm H2O (16). The closing pressure is higher in the presence of small airway obstruction (e.g., in COPD) and reduced lung compliance (e.g., in ARDS), and this results in more extensive airspace collapse at end-expiration. This has two adverse consequences: (a) impaired gas exchange from atelectasis, and (b) atelectrauma from repetetive closing and opening of the distal airspaces (17,18).
The risk of alveolar collapse at end-expiration can be eliminated by preventing the airway pressure from falling below the closing pressure at the end of expiration. This is achieved by creating a positive pressure in the airways at the end of expiration that is equivalent to the closing pressure. This positive end-expiratory pressure (PEEP) is created by a pressure-relief valve in the expiratory limb of the ventilator circuit that allows exhalation to proceed until the pressure falls to the preselected level, and this pressure is then maintained for the duration of expiration. Since it is not possible to identify the closing pressure in the clinical setting, a PEEP level of 5–7 cm H2O is used to prevent alveolar collapse in all patients.
Inflation Pressures
The influence of PEEP on inflation pressures is illustrated in Figure 26.4. Note that the addition of PEEP moves the inflation pressure waveform upward, and results in a higher peak alveolar pressure and a higher mean airway pressure. The following statements are relevant in this regard.
1. The effects of PEEP are not related to the PEEP level, but are determined by the influence of PEEP on the peak alveolar pressure and the mean airway pressure.
2. The change in peak alveolar pressure determines the influence of PEEP on alveolar ventilation (and hence arterial oxygenation), and also determines the risk of alveolar overdistension and volutrauma (see page 494).
3. The change in mean airway pressure determines the influence of PEEP on cardiac output.
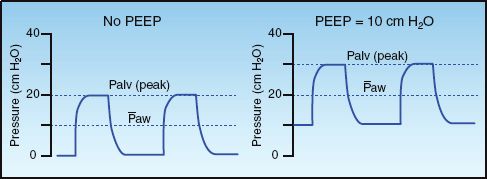
FIGURE 26.4 Airway pressure waveforms during pressure control ventilation showing the effects of positive end-expiratory pressure (PEEP) on the peak alveolar pressure (Palv) and the mean airway pressure (Paw).
Alveolar Recruitment
While low levels of PEEP (5–10 cm H2O) help to prevent the collapse of distal airspaces, high levels of PEEP (20–30 cm H2O) help to reopen distal airspaces that are persistently collapsed. This effect is demonstrated in Figure 26.5 (19). The thoracic CT image on the left shows consolidation in the posterior (dependent) regions of both lungs, and this disappears after the addition of PEEP (at 19 cm H2O). This effect is known as alveolar recruitment, and it increases the available surface area in the lungs for gas exchange (19,20).
Recruitable Lung Volume
The addition of PEEP may not result in alveolar recruitment, but instead can overdistend alveoli in normal lung regions, which increases the risk of ventilator-induced lung injury. The volume of “recruitable lung” (i.e., areas of atelectasis that can be aerated) determines if PEEP will promote alveolar recruitment or alveolar overdistension; i.e., if there is a significant volume of recruitable lung, then PEEP will promote alveolar recruitment and improve pulmonary gas exchange, but if there is a negligible volume of recruitable lung, then PEEP will promote alveolar overdistension and increase the risk of ventilator-induced lung injury.
The volume of recruitable lung varies widely in individual patients (from 2% to 25% of total lung volume) (20). Although it is not possible to measure recruitable lung volume reliably, the following measures can be used to determine if PEEP is promoting alveolar recruitment (favorable response) or promoting alveolar overdistension (unfavorable response).
LUNG COMPLIANCE: When PEEP is promoting alveolar recruitment, the compliance (distensibility) of the lungs will increase, but when PEEP is overdistending alveoli in normal lung regions, lung compliance will decrease. The measurement of lung compliance during mechanical ventilation is described in Chapter 25 (see pages 492–493).
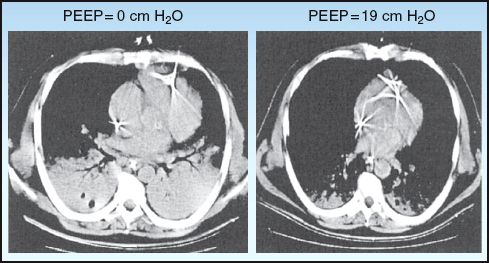
FIGURE 26.5 Thoracic CT images from a patient with ARDS showing the influence of PEEP on lung aeration (alveolar recruitment). Images from Reference 19.
PAO2/FIO2 RATIO: The relationship between the arterial PO2 and the fractional concentration of inspired oxygen, as expressed by the PaO2/FIO2 ratio, is a measure of the efficiency of gas exchange in the lungs. When PEEP promotes alveolar recruitment, the PaO2/FIO2 ratio will increase, and when PEEP does not promote alveolar recruitment, the PaO2/FIO2 ratio will remain unchanged or will decrease. An example of a beneficial response in the PaO2/FIO2 ratio (indicating alveolar recruitment) is shown in Figure 26.6.
Systemic O2 Transport
The beneficial effects of PEEP on arterial oxygenation may not be accompanied by a similar benefit in systemic oxygen transport, as described next.
Cardiac Output
The influence of positive pressure ventilation on cardiac performance is described at the end of Chapter 25 (see pages 499–501). PEEP magnifies these effects, particularly the tendency for positive pressure ventilation to decrease cardiac output. There are several mechanisms whereby PEEP can decrease cardiac output, including (a) impaired venous return, (b) decreased ventricular compliance, (c) increased right ventricular afterload, and (d) external constraint of the ventricles (21,22). These effects are more prominent in the presence of hypovolemia.
Oxygen Delivery
PEEP-induced decreases in cardiac output can erase the beneficial effects of PEEP on alveolar recruitment. This is illustrated by the following equation for systemic oxygen delivery (DO2). (see page 178 for the derivation of this equation.)
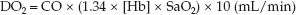 (26.1)
(26.1)
Thus, PEEP can promote alveolar recruitment and increase arterial oxygenation (SaO2), but systemic O2 delivery may not improve if PEEP also decreases the cardiac output (CO). The opposing effects of PEEP on arterial oxygenation and cardiac output are demonstrated in Figure 26.6 (23).
BEST PEEP: The optimal benefit from PEEP occurs when an increase in arterial oxygenation is accompanied by an increase in systemic O2 delivery. Therefore, the optimal or best PEEP for an individual patient is the level of PEEP that results in the greatest increase in systemic O2 delivery (24). Best PEEP can be determined by measuring the systemic O2 delivery at increasing levels of PEEP. Unfortunately, the decline in popularity of the pulmonary artery catheter (which provides the most reliable measurements of cardiac output) has also decreased the popularity of best PEEP determinations.
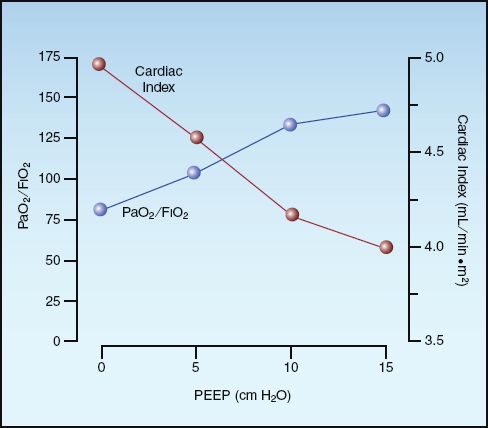
FIGURE 26.6 The opposing effects of positive end-expiratory pressure (PEEP) on arterial oxygenation (PaO2/FIO2) and cardiac index in patients with ARDS. Data from Reference 23.
VENTILATOR SETTINGS
When mechanical ventilation is initiated, the respiratory therapist will ask you for the following parameters: (a) mode of ventilation, (b) tidal volume, (c) respiratory rate, (d) PEEP level, and (e) inspired O2 concentration. The following is a list of suggestions for setting up mechanical ventilation.
Assist-Control Ventilation
1. Select assist-control as the initial mode of ventilation.
2. It may be necessary to switch to synchronized IMV in patients who are breathing too rapidly in the assist-control mode (see later).
Volume vs. Pressure Control
1. The use of volume control or pressure control is largely a matter of personal preference, although some patients breathe more comfortably with pressure control.
2. A suitable compromise is the pressure-regulated volume control mode of ventilation (PRVC), which allows for control of the tidal volume, but limits the airway pressures.
Tidal Volume
The following recommendations are from the lung protective ventilation protocol, which is summarized in Table 25.1 (see page 498).
1. Select an initial tidal volume of 8 mL/kg using predicted body weight. (The formulas for calculating predicted body weight are in Table 25.1.)
2. Reduce the tidal volume to 6 mL/kg over the next 2 hours, if possible.
3. Monitor the peak alveolar pressure and keep it ≤30 cm H2O (to limit the risk of volutrauma).
a. In the volume control mode, the peak alveolar pressure is the end-inspiratory occlusion pressure, also called the plateau pressure (see pages 489–491).
b. In the pressure control mode, the peak alveolar pressure is the end-inspiratory airway pressure (as long as there is no flow at the end of inspiration).
Inspiratory Flow Rate
1. Select an inspiratory flow rate of 60 L/min if the patient is breathing quietly or has no spontaneous respirations.
2. Use higher inspiratory flow rates (e.g., ≥80 L/min) for patients with respiratory distress or a high minute ventilation (≥10 L/min).
I:E Ratio
1. The I:E ratio should be ≥1:2.
2. If the I:E ratio is <1:2, the options for increasing the I:E ratio include: (a) increasing the inspiratory flow rate, (b) decreasing the tidal volume, or (c) decreasing the respiratory rate, if possible.
Respiratory Rate
1. If the patient has no spontaneous respirations, set the respiratory rate to achieve your estimate of the patient’s minute ventilation just prior to intubation, but do not exceed 35 breaths/minute.
2. If the patient is triggering each ventilator breath, set the machine rate just below the patient’s spontaneous respiratory rate.
3. After 30 minutes, check the arterial PCO2, and adjust the respiratory rate, if necessary, to achieve the desired PCO2.
4. For patients who are breathing rapidly and have an acute respiratory alkalosis or evidence of occult PEEP, consider switching to synchronized IMV (SIMV) as the mode of ventilation.
PEEP
1. Set the initial PEEP to 5 cm H2O to prevent the collapse of distal airspaces at end-expiration.
2. Further increases in PEEP may be required if either of the following conditions is present: (a) a toxic level of inhaled oxygen (>60%) is required to maintain adequate oxygenation (SaO2 ≥90%), or (b) hypoxemia is refractory to oxygen therapy.
Occult PEEP
1. Check the flow rate at the end of expiration to detect the presence of intrinsic or occult PEEP (see Figure 24.5 on page 479).
2. If the expiratory flow rate has not returned to the zero at end-expiration (indicating the presence of occult PEEP), attempt to prolong the time for expiration by increasing the I:E ratio using the maneuvers described previously.
3. If increasing the I:E ratio is not possible or is not successful, then measure the level of occult PEEP with the end-inspiratory occlusion method, and add extrinsic PEEP at a level that is just below the occult PEEP. (The rationale for this maneuver is explained in Chapter 28.)
A FINAL WORD
Loss of Focus
Considering that the human capacity to store and process information is limited to 4 variables (25), the 174 documented modes of ventilatory assistance mentioned in the introduction represents an incomprehensible load of information. What seems to be lost in this maze of technology is the simple fact that mechanical ventilation is a temporary support measure for acute respiratory failure, and is not a treatment modality for lung disease. Therefore, if we want to improve outcomes in ventilator-dependent patients, less attention should be given to the knobs on ventilators, and more attention should be directed at the diseases that create ventilator dependency.
REFERENCES
Introduction
1. Cairo JM, Pilbean SP. Mosby’s Respiratory Care Equipment. 8th ed. St. Louis: Mosby Elsevier; 2010.
2. The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342(18):1301–1308.
3. Mireles-Cabodevila E, Hatipoglu U, Chatburn RL. A rational framework for selecting modes of ventilation. Respir Care 2013; 58:348–366.
The Ventilator Breath
4. Petrucci N, Iacovelli W. Ventilation with lower tidal volumes versus traditional tidal volumes for acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2004; (2):CD003844.
5. Yang SC, Yang SP. Effects of inspiratory flow waveforms on lung mechanics, gas exchange, and respiratory metabolism in COPD patients during mechanical ventilation. Chest 2002; 122:2096–2104.
6. Kallet RH, Campbell AR, Alonzo JA, et al. The effects of pressure control versus volume control on patient work of breathing in acute lung injury and acute respiratory distress syndrome. Respir Care 2000; 45:1085–1096.
Assist-Control Ventilation
7. Hess DR, Kacmarek RM. Physiologic effects of mechanical ventilation. In: Essentials of Mechanical Ventilation. New York, McGraw-Hill, 1996:1–10.
8. Leung P, Jubran A, Tobin MJ. Comparison of assisted ventilator modes on triggering, patients’ effort, and dyspnea. Am J Respir Crit Care Med 1997; 155:1940–1948.
9. Laureen H, Pearl R. Flow triggering, pressure triggering, and autotriggering during mechanical ventilation. Crit Care Med 2000; 28:579–581.
Intermittent Mandatory Ventilation
10. Shelledy DC, Rau JL, Thomas-Goodfellow L. A comparison of the effects of assist-control, SIMV, and SIMV with pressure-support on ventilation, oxygen consumption, and ventilatory equivalent. Heart Lung 1995; 24:67–75.
11. Singh I, Pinsky MR. Heart-lung interactions. In Papadakos PJ, Lachmann B, eds. Mechanical ventilation: clinical applications and pathophysiology. Philadelphia: Saunders Elsevier, 2008:173–184.
12. Mathru M et al. Hemodynamic responses to changes in ventilatory patterns in patients with normal and poor left ventricular reserve. Crit Care Med 1982; 10:423–426.
Pressure-Support Ventilation
13. Hess DR. Ventilator waveforms and the physiology of pressure support ventilation. Respir Care 2005; 50:166–186.
14. Jubran A, Grant BJ, Duffner LA, et al. Effect of pressure support vs. unassisted breathing through a tracheostomy collar on weaning duration in patients requiring prolonged mechanical ventilation: a randomized trial. JAMA 2013; 309:671–677.
15. Caples SM, Gay PC. Noninvasive positive pressure ventilation in the intensive care unit: a concise review Crit Care Med 2005; 33:2651–2658.
Positive End-Expiratory Pressure
16. Hedenstierna G, Bindslev L, Santesson J. Pressure-volume and airway closure relationships in each lung of anesthetized man. Clin Physiol 1981; 1:479–493.
17. Muscedere JG, Mullen JBM, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 1994; 149:1327–1334.
18. Gattinoni L, Protti A, Caironi P, Carlesso E. Ventilator-induced lung injury: the anatomical and physiological framework. Crit Care Med 2010; 38(Suppl):S539–S548.
19. Barbas CSV. Lung recruitment maneuvers in acute respiratory distress syndrome and facilitating resolution. Crit care Med 2003; 31(Suppl):S265–S271.
20. Gattinoni L, Cairon M, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 2006; 354:1775–1786.
21. Schmitt J-M, Viellard-Baron A, Augarde R, et al. Positive end-expiratory pressure titration in acute respiratory distress syndrome patients: Impact on right ventricular outflow impedance evaluated by pulmonary artery Dop-pler flow velocity measurements. Crit Care Med 2001; 29:1154–1158.
22. Takata M, Robotham JL. Ventricular external constraint by the lung and pericardium during positive end-expiratory pressure. Am Rev Respir Dis 1991; 43:872–875.
23. Gainnier M, Michelet P, Thirion X, et al. Prone position and positive end-expiratory pressure in acute respiratory distress syndrome. Crit Care Med 2003; 31:2719–2726.
24. Punt CD, Schreuder JJ, Jansen JR, et al. Tracing best PEEP by applying PEEP as a RAMP. Intensive Care Med 1998; 24:821–828.
A Final Word
25. Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci 2001; 24:87–114.

Full access? Get Clinical Tree








